一种针对结直肠癌RhoA的Cys16共价抑制剂
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
RhoA是一种关键的癌症驱动因子和潜在的结直肠癌治疗靶点,但在临床上尚未得到证实。利用基于活性的蛋白谱分析(ABPP)和质谱分析(MS),我们鉴定出了CL16,一种针对RhoA亚家族中独特的Cys16的共价抑制剂,它比其他Rho家族蛋白具有更高的特异性。Cys16邻近核苷酸结合口袋区和开关区,这对RhoA功能至关重要。CL16的结合有效地破坏了GTP的结合,抑制了CRC细胞中RhoA的活性,通过细胞周期阻滞和凋亡导致CRC细胞的细胞毒性杀伤。在小鼠CRC模型中,CL16表现出较强的抗肿瘤和抗转移作用,促进T细胞向肿瘤微环境浸润,无明显毒性。我们的研究结果表明,可药物Cys16共价靶向RhoA为CRC治疗提供了一种很有前景的策略,为开发特异性的RhoA抑制剂用于临床应用奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
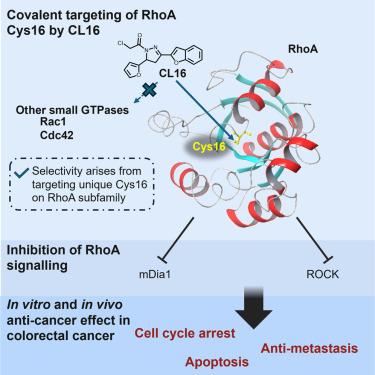
A covalent inhibitor targeting Cys16 on RhoA in colorectal cancer
RhoA is a key cancer driver and potential colorectal cancer (CRC) therapy target but remains undrugged clinically. Using activity-based protein profiling (ABPP) and mass spectrometry (MS), we identified CL16, a covalent inhibitor targeting the unique Cys16 on RhoA subfamily, which confers high specificity over other Rho family proteins. Cys16 is adjacent to the nucleotide-binding pocket and switch regions, which are critical for RhoA function. The binding by CL16 effectively disrupts GTP binding and inhibits RhoA activity in CRC cells, leading to cytotoxic killing of CRC cells through cell-cycle arrest and apoptosis. In mouse CRC models, CL16 exhibits strong antitumor and antimetastatic effects, promotes T cell infiltration into the tumor microenvironment, and shows no observable toxicity. Our findings suggest that covalent targeting of the druggable Cys16 on RhoA offers a promising strategy for CRC treatment, providing a foundation for developing specific RhoA inhibitors for clinical application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


