改善阿片镇痛:通过联合治疗调节mu-阿片受体磷酸化状态
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
阿片类镇痛药通常用于减轻病理性疼痛。除了镇痛作用外,这种药物治疗方案因其诱导不良反应的能力而闻名,包括阿片类药物诱导的痛觉过敏(OIH)和镇痛耐受性。因此,迫切需要新的有效的治疗策略来改善阿片类药物镇痛,同时减轻副作用,以确保患者的安全。目前,增加阿片类镇痛药的获益/风险概况的努力,特别是联合药物治疗,是非有希望的,并取得了很大进展。与单一治疗相比,阿片类镇痛药和某些类型的非阿片类镇痛药联合使用可减少所需的镇痛剂量并减少剂量限制性副作用。然而,对协同镇痛的机制知之甚少。受羧基末端磷酸化控制的μ -阿片受体(μOR)的脱敏和内化限制了阿片药物的疗效,是引发镇痛耐受和OIH的基础。在这里,我们调查了阿片类药物和非阿片类药物联合治疗疼痛的协同效应;这些组合通过调节μOR磷酸化和去磷酸化来改善阿片镇痛并减少副作用。此外,我们讨论了这些非阿片受体及其制剂如何参与蛋白激酶介导的μOR磷酸化和蛋白磷酸酶介导的μOR去磷酸化的调节。本文章由计算机程序翻译,如有差异,请以英文原文为准。
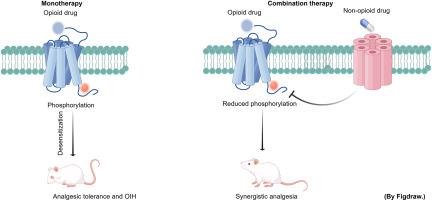
Improving opioid analgesia: Modulation of the mu-opioid receptor phosphorylation state via combination therapy
Opioid analgesics are commonly prescribed to mitigate pathological pain. In addition to its analgesic effect, this pharmaceutical treatment program is well-known for its ability to induce adverse effects, including opioid-induced hyperalgesia (OIH) and analgesic tolerance. Thus, novel effective therapeutic strategies are urgently needed to improve opioid analgesia while mitigating side effects to ensure patient safety. Currently, efforts to increase the benefit/risk profiles of opioid analgesics, particularly combination pharmacotherapy, are highly promising and have made great progress. When combined, opioid analgesics and certain types of nonopioids have been found to reduce the required analgesic dose and decrease dose-limiting side effects compared with monotherapy. However, little is known about the mechanisms that underlie synergistic analgesia. The desensitization and internalization of the mu-opioid receptor (μOR), which is controlled by carboxyl-terminal phosphorylation, limits the efficacy of opioid drugs and underlies the initiation of analgesic tolerance and OIH. Here, we survey the synergistic effects of opioid and nonopioid combinations in pain treatment; these combinations improve opioid analgesia and reduce side effects by modulating μOR phosphorylation and dephosphorylation. Furthermore, we discuss how these nonopioid receptors and their agents are involved in the regulation of protein kinase-mediated μOR phosphorylation and protein phosphatase-mediated μOR dephosphorylation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


