天然受损的芳香3-酮酰基辅酶a侧链缩短揭示了植物苯乙酮的生物合成途径
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
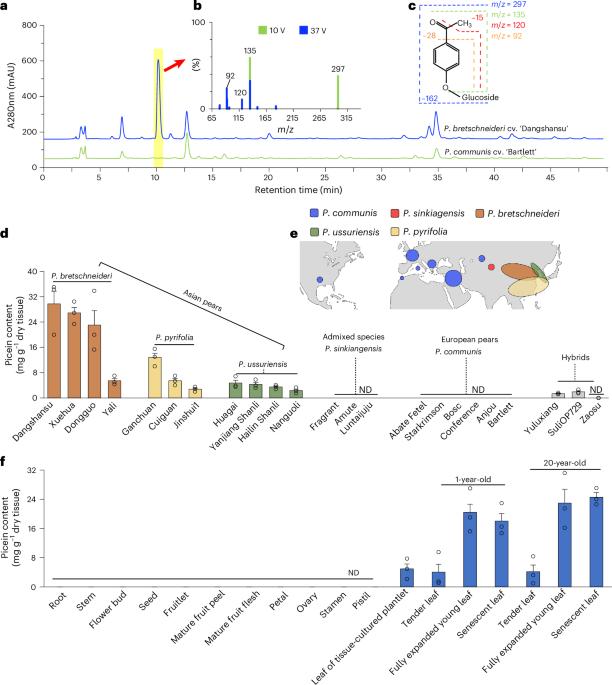
苯乙酮在植物-植物、植物-昆虫、植物-微生物甚至动物-昆虫的相互作用中发挥着不同的作用,在系统发育上较远的植物和真菌中表现出分散分布。然而,植物中苯乙酮生物合成的酶基础尚不清楚。本文以梨(Pyrus)为研究对象,研究了4- coumaryl - coa合成4-羟基苯乙酮糖苷的完整生物合成途径。我们证明,在某些梨品种中,苯乙酮片段源于芳香3-酮酰基辅酶a中间体侧链缩短反应受损,这是苯甲酸β-氧化生物合成的关键步骤。这种损伤是由过氧化物酶体3-酮酰基辅酶a硫酶的功能突变丧失引起的。积累的芳香3-酮酰基辅酶a随后被硫酯酶水解,并进行自发脱羧,产生苯乙酮部分。这种罕见的代谢现象表明,除了新功能化外,功能缺失突变也可以驱动植物次生代谢的多样化。正向遗传方法对于揭示植物中这种“隐藏的”或隐性的途径是强有力的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Naturally impaired side-chain shortening of aromatic 3-ketoacyl-CoAs reveals the biosynthetic pathway of plant acetophenones
Acetophenones, which show scattered distribution across phylogenetically distant plants and fungi, play diverse roles in plant–plant, plant–insect, plant–microbiome and even animal–insect interactions. However, the enzymatic basis of acetophenone biosynthesis in plants remains unknown. Here we elucidate the complete biosynthetic pathway of picein (4-hydroxyacetophenone glucoside) from 4-coumaroyl-CoA using pear (Pyrus) as a study system. We demonstrate that in certain pear cultivars, the acetophenone moiety originates from an impaired side-chain shortening reaction of an aromatic 3-ketoacyl-CoA intermediate, a key step in the β-oxidative biosynthesis of benzoic acid. This impairment results from a loss-of-function mutation in a peroxisomal 3-ketoacyl-CoA thiolase. The accumulated aromatic 3-ketoacyl-CoA is subsequently hydrolysed by a thioesterase and undergoes spontaneous decarboxylation to yield the acetophenone moiety. This rare metabolic phenomenon highlights that not only neofunctionalization but also loss-of-function mutations can drive diversification in plant secondary metabolism. Forward genetic approaches are powerful to shed light on such ‘hidden’ or recessive pathways in plants. Using pear as a study system, the biosynthetic pathway of acetophenones has been elucidated: naturally impaired side-chain shortening of aromatic 3-ketoacyl-CoAs leads them to undergo hydrolysis, followed by decarboxylation, to yield acetophenones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


