厄伐环素在并发腹腔内感染和社区获得性细菌性肺炎患者中的群体药代动力学和药效学:对固定剂量治疗方案的支持
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-09-01
DOI:10.1016/j.ijantimicag.2025.107603
引用次数: 0
摘要
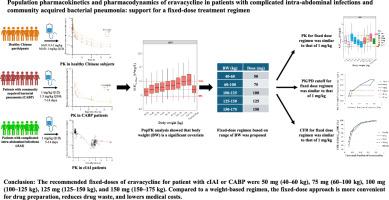
依拉瓦环素是一种四环素,用于治疗复杂性腹腔内感染(cIAI),并具有治疗社区获得性细菌性肺炎(CABP)的潜力。批准的cIAI方案为1mg/kg (Q12h)。然而,研究报告了基于体重(BW)和药物浪费的药物制备的不便,因为规格为每瓶50毫克。基于群体药代动力学/药效学(PK/PD)建模和模拟的固定剂量方案可以解决这些局限性。在中国健康受试者和cIAI和CABP患者中进行了三项临床试验。采用非线性混合效应建模方法建立了PopPK模型。根据蒙特卡洛模拟,推荐体重为40-175 kg的患者采用固定剂量方案。计算目标达到概率和累计反应分数(CFR)。总共有79名参与者参与了这项研究。体重、性别、白蛋白水平和受试者类型是影响PK的协变量。当患者接受固定剂量(AUC0-12h)时,体重60公斤的患者接受1 mg/kg的依瓦环素治疗时,ss为80-125%。固定剂量方案的PK/PD临界值和CFR与1 mg/kg方案接近。推荐的固定剂量(体重范围)为50毫克(40-60公斤)、75毫克(60-100公斤)、100毫克(100-125公斤)、125毫克(125-150公斤)和150毫克(150-175公斤)。由于没有收集实际的PK数据,因此对于体重为137kg的患者应谨慎使用。尽管如此,与重量计量相比,固定计量更方便药物制备,避免药物浪费,降低医疗成本。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Population pharmacokinetics and pharmacodynamics of eravacycline in patients with complicated intra-abdominal infections and community acquired bacterial pneumonia: Support for a fixed-dose treatment regimen
Eravacycline is a tetracycline used for the treatment of complicated intra-abdominal infections (cIAI) and has the potential to treat community-acquired bacterial pneumonia (CABP). The approved regimen for cIAI is 1 mg/kg (Q12h). However, studies have reported the inconvenience of drug preparation based on body weight (BW) and wastage of the drug, because the specification is 50 mg per vial. A fixed-dose regimen based on population pharmacokinetic/pharmacodynamic (PK/PD) modeling and simulations may address these limitations. Three clinical trials were performed in healthy Chinese participants and patients with cIAI and CABP. A PopPK model was developed using nonlinear mixed-effects modeling. A fixed-dose regimen in patients with a BW of 40–175 kg was recommended by the Monte Carlo simulation. The probability of target attainment and the cumulative fraction of response (CFR) were calculated. Overall, 79 participants were included in the study. BW, sex, albumin level, and subject type were covariates on PK. When patients received a fixed-dose, AUC0-12 h,ss was 80–125% in patients with BW 60 kg receiving 1 mg/kg eravacycline. The PK/PD cutoff and CFR for the fixed-dose regimens were close to those of 1 mg/kg. The recommended fixed-doses (body weight range) were 50 mg (40–60 kg), 75 mg (60–100 kg), 100 mg (100–125 kg), 125 mg (125–150 kg), and 150 mg (150–175 kg). This should be used with caution in patients with BW >137 kg because the actual PK data were not collected. Nonetheless, fixed dosing is more convenient for drug preparation, avoids drug waste, and reduces medical costs compared with weight-based dosing.
Trial Registration Number: ChiCTR1900022906, ChiCTR1900022060, and ChiCTR2200055666.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


