评估罗氏cobas MTB和MTB- rif /INH检测结核分枝杆菌复体和对异烟肼和利福平的耐药性:一项前瞻性,多中心诊断准确性研究。
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-09-01
DOI:10.1016/j.ijantimicag.2025.107605
引用次数: 0
摘要
目的:评价罗氏cobas MTB和MTB-RIF/INH检测结核分枝杆菌复合体(MTBC)及对异烟肼(INH)和利福平(RIF)耐药的诊断效果。方法:本研究于2023年9月至2024年6月在台湾进行。临床标本采集自推定结核病(TB)的成年患者。所有样品均进行抗酸染色、培养、Xpert MTB/RIF Ultra检测和cobas MTB检测。MTB- rif /INH试验是对cobas MTB试验呈阳性的标本进行反射试验。对台湾省疾病预防控制中心(TCDC)的37株MTBC对照菌株进行耐药性检测。使用全基因组测序来解决与表型药敏试验(pDST)的差异。结果:共分析了378例成人患者的425份临床样本。在392份呼吸道样本中,53份MTBC培养阳性。cobas MTB检测MTBC的灵敏度为98.1%(95%可信区间[CI], 89.9% ~ 100%),特异性为95.9% (95% CI, 93.2% ~ 97.7%)。采用37株TCDC菌株进行耐药检测。对于RIF耐药,cobas MTB-RIF/INH试验正确鉴定了所有11株表型耐药菌株。26株表型敏感菌株中,正确鉴定25株,假阳性1株,总体准确率97.3%。对INH耐药(0.2 μg/mL),鉴定出耐药株16株,敏感株19株,假阴性2株,准确率为94.6%。结论:罗氏cobas MTB和MTB- rif /INH检测在检测MTBC和耐药方面具有较高的准确性,支持其作为临床实践中可靠的诊断工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
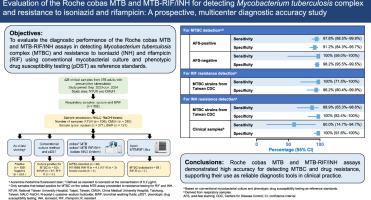
Evaluation of the Roche cobas MTB and MTB-RIF/INH for detecting Mycobacterium tuberculosis complex and resistance to isoniazid and rifampicin: A prospective, multicenter diagnostic accuracy study
Objectives
To evaluate the diagnostic performance of Roche cobas MTB and MTB-RIF/INH assays for detecting Mycobacterium tuberculosis complex (MTBC) and resistance to isoniazid (INH) and rifampicin (RIF).
Methods
This multicentre study was conducted in Taiwan between September 2023 and June 2024. Clinical specimens were collected from adult patients with presumptive tuberculosis (TB). All samples underwent acid-fast staining, culture, Xpert MTB/RIF Ultra assay and cobas MTB assays. The MTB-RIF/INH assay was conducted as a reflex test for specimens that tested positive with the cobas MTB assay. Thirty-seven MTBC control strains from the Taiwan Centers for Disease Control (TCDC) were also analysed to assess drug resistance detection. Whole genome sequencing was used to resolve discrepancies with phenotypic drug susceptibility testing (pDST).
Results
A total of 425 clinical samples from 378 adult patients were analysed. Among 392 respiratory samples, 53 were culture-positive for MTBC. cobas MTB showed 98.1% sensitivity (95% confidence interval [CI], 89.9%–100%) and 95.9% specificity (95% CI, 93.2%–97.7%) for MTBC detection. Drug resistance detection was evaluated using 37 TCDC stains. For RIF resistance, the cobas MTB-RIF/INH assay correctly identified all 11 phenotypically resistant strains. Among the 26 phenotypically susceptible strains, 25 were correctly identified, with 1 false-positive result (overall accuracy: 97.3%). For INH resistance (0.2 µg/mL), the assay identified 16 resistant and 19 susceptible strains, with 2 false negatives (accuracy: 94.6%).
Conclusions
Roche cobas MTB and MTB-RIF/INH assays demonstrated high accuracy for detecting MTBC and drug resistance, supporting their use as reliable diagnostic tools in clinical practice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


