活化SAMHD1的dNTP三磷酸水解酶连续测定。
IF 2.5
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
无菌α基序和组氨酸-天冬氨酸结构域蛋白1 (SAMHD1)是人类三磷酸单酯水解酶家族(dNTP + H2O→dN + PPPi)中唯一的成员。SAMHD1的dNTPase活性抑制DNA合成,导致细胞周期阻滞,限制病毒复制。SAMHD1复杂的变构调节机制和缺乏直接光谱信号的反应给其动力学分析和抑制剂的发现带来了挑战。我们描述了一个连续监测SAMHD1磷酸酶活性在其激活的生理状态。该分析使用顺序组装来产生活性四聚体形式的酶。两种磷酸酶将无机三磷酸(PPPi)转化为无机磷酸(Pi)。释放的Pi与7-甲基-6-硫鸟嘌呤核苷磷酸化酶反应,提供灵敏的连续分光光度测定。该分析适用于96微孔板格式,以提供SAMHD1活性的连续测量。该试验以SAMHD1抑制剂为基准。该试验的Z-prime值为> 0.90,可用于高通量筛选SAMHD1抑制剂,并表征新抑制剂的变构或催化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
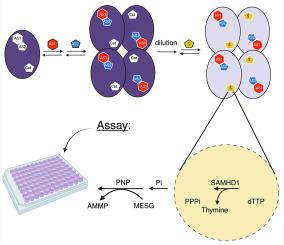
Continuous assay for the dNTP triphosphohydrolase of activated SAMHD1
Sterile alpha motif and histidine-aspartate domain-containing protein 1 (SAMHD1) is the only member of the triphosphoric monoester hydrolase family in humans (dNTP + H2O → dN + PPPi). The dNTPase activity of SAMHD1 inhibits DNA synthesis, resulting in cell-cycle arrest and restricting viral replication. The complex allosteric regulation mechanism of SAMHD1 and a reaction that lacks a direct spectroscopic signal make its kinetic analysis and inhibitor discovery challenging. We describe a continuous assay for monitoring SAMHD1 phosphatase activity in its activated physiological state. The assay uses a sequential assembly to generate the active tetrameric form of the enzyme. Two phosphatases convert inorganic triphosphate (PPPi) to inorganic phosphate (Pi). The released Pi reacts with the 7-methyl-6-thioguanosine and purine nucleoside phosphorylase to provide a sensitive continuous spectrophotometric assay. The assay is suitable for 96-microwell plate formats to provide a continuous measurement of SAMHD1 activity. The assay is benchmarked with inhibitors of SAMHD1. With a Z-prime value > 0.90, the assay can be used for high-throughput screening of inhibitors for SAMHD1 and characterizing the allosteric or catalytic activity of the new inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


