电压门控钙通道在缺血/再灌注中的作用和行为
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
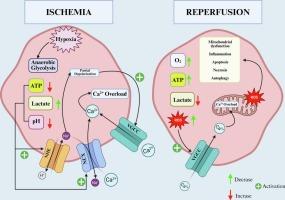
缺血/再灌注(I/R)损伤是由钙失衡、氧化应激、线粒体功能障碍和炎症过程等多方面机制复杂相互作用引起的病理状态。电压门控钙通道(VGCCs)在这一发病机制中发挥关键作用,通过调节钙流入细胞,从而启动一系列有害的细胞内事件。在缺血阶段,ATP储备的消耗导致钙转运系统的功能障碍;在再灌注阶段,活性氧(ROS)对vgc的刺激加剧了细胞内钙超载。这种积累触发了线粒体通透性过渡孔的打开,增加了ROS的产生,并激活了细胞死亡途径,如凋亡、坏死和铁下垂。本综述详细探讨了vgc的结构亚型和生理功能,同时广泛研究了vgc在I/R条件下的行为,包括心血管、神经、肾脏和生殖系统。这篇综述的重点是L型、T型、N型和r型vgc的不同作用,并检查了组织特异性和异构体特异性药物阻断策略的最新发现。实验研究证明了VGCC抑制剂(如尼莫地平、米贝弗拉迪和snx -111)的保护作用,并对其翻译局限性进行了严格评估。通过将最新的机制见解与临床前和早期临床数据相结合,本综述强调了vgc是预防I/R损伤的有前途的分子靶点。未来的治疗策略应侧重于异构体特异性靶向、时间依赖性给药和器官定向制剂,以提高疗效和安全性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The role and behavior of voltage-gated calcium channels in ischemia/reperfusion
Ischemia/reperfusion (I/R) injury is a pathological condition that arises from the complex interplay of multifaceted mechanisms such as calcium imbalance, oxidative stress, mitochondrial dysfunction, and inflammatory processes. Voltage-gated calcium channels (VGCCs) play a critical role in this pathogenesis by regulating calcium influx into the cell, thereby initiating a cascade of detrimental intracellular events. During the ischemic phase, depletion of ATP reserves leads to the dysfunction of calcium transport systems; in the reperfusion phase, the stimulation of VGCCs by reactive oxygen species (ROS) intensifies intracellular calcium overload. This accumulation triggers the opening of mitochondrial permeability transition pores, amplifies ROS production, and activates cell death pathways such as apoptosis, necrosis, and ferroptosis.
This comprehensive review explores the structural subtypes and physiological functions of VGCCs in detail while broadly investigating their behavior under I/R conditions across various organ systems, including the cardiovascular, neurological, renal, and reproductive systems. The review focuses on the distinct roles of L-, T-, N-, and R-type VGCCs and examines current findings on tissue- and isoform-specific pharmacological blockade strategies. Experimental studies demonstrating the protective effects of VGCC inhibitors—such as nimodipine, mibefradil, and SNX-111—are critically evaluated along with their translational limitations.
By integrating up-to-date mechanistic insights with preclinical and early clinical data, this review highlights VGCCs as promising molecular targets for preventing I/R injury. Future therapeutic strategies should focus on isoform-specific targeting, time-dependent administration, and organ-directed formulations to enhance efficacy and safety.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


