可回收深共熔溶剂(DES)辅助无金属单锅合成氮杂蒽醌支架及其对接(SAR)研究、生物活性和荧光性能
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了一种无金属、高效的绿色合成方法,以芳香醛、活性亚甲基底物(2a/2b)和2-氨基萘-1,4-二酮(3a)为多组分反应,采用可回收的环保尿素-锌cl2(3:1)深度共晶溶剂(DES)作为催化剂和反应介质,合成取代氮杂蒽醌支架。该方案有效地合成了杂化氮杂蒽醌,表现出高原子经济性和区域选择性,同时取得了显著的产量和可扩展性克数量。值得注意的优点包括使用廉价的起始材料,与各种支架的相容性,以及采用深共晶溶剂作为绿色有机催化剂和溶剂的创新环保方法。此外,对合成的基态进行了光物理表征分析,发现化合物4d和4h分别在562和546处表现出强烈的发射带,并伴有较大的Stokes位移(117和80 nm)。此外,生物学评价突出了显著的抗真菌和抗菌活性,化合物4b、4d、4e、4f、4g、4l和4b分别对标准药物(庆大霉素用于抗菌,制霉菌素用于抗真菌)显示出更大的抑制区。此外,利用Maestro Schrodinger软件中的Glide工具,对合成的分子进行了分子对接研究,对含有SARS-CoV-2蛋白的remdesivir(抗病毒药物)的抗病毒活性进行了评估,结果表明,化合物4a具有- 5.64 kcal/mol的滑翔分数,- 41.27 kcal/mol的滑翔能量和89.79%的生物利用度,表明其具有作为抗病毒分子的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
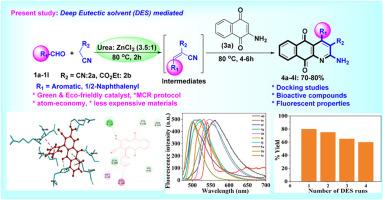
Recyclable deep eutectic solvent (DES) assisted metal free one-pot synthesis of aza-anthraquinone scaffolds, and their docking (SAR) studies, biological activity and fluorescent properties
We report a metal-free, efficient green synthetic approach for the synthesis of substituted Aza-anthraquinone scaffolds from the multicomponent reaction of aromatic aldehydes, active methylene substrates (2a/2b), and 2-aminonaphthalene-1,4-dione (3a) employing a recyclable eco-friendly Urea-ZnCl2 (3:1) deep eutectic solvent (DES) as catalyst cum reaction medium. This protocol effectively synthesizes functionalized Aza-anthraquinone hybrids, exhibiting high atom economy and exclusive regioselectivity, while achieving remarkable yields and demonstrating scalability to gram quantities. Notable benefits include the utilization of inexpensive starting materials, compatibility with various scaffolds, and an innovative eco-friendly methodology that employs deep eutectic solvents as green organocatalysts and solvents. Further, synthesized substates were analyzed photophysical characterization that revealed promising photophysical properties, with compounds 4d and 4h exhibiting intense emission bands at 562 and 546, respectively, accompanied by large Stokes shifts (117 and 80 nm). Additionally, biological evaluation highlighted significant antifungal and antibacterial activities, with compounds 4b, 4d, 4e, 4f, 4g, 4l, and 4b respectively displaying greater inhibition zones against standard drugs (Gentamicin for antibacterial, Nystatin for antifungal assays). Additionally, molecular docking studies evaluated synthesized molecules, using Glide tool in the Maestro Schrodinger suite, for antiviral activity against remdesivir (antiviral drug) with SARS-CoV-2 protein, which indicated that compound 4a possesses a glide score of −5.64 kcal/mol, glide energy of −41.27 kcal/mol, and 89.79 % bioavailability, suggesting it's potential as an antiviral molecule against SARS-CoV-2 protein.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


