原位锂化和形状修饰的V2O5纳米棒作为高性能锂离子电池和锂离子电容器的负极材料
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
五氧化二钒(V2O5)是下一代锂离子电池(LIBs)和锂离子电容器(lic)极具发展前景的电极材料,但其结构和电化学稳定性差限制了其性能。本研究提出了一种两步水热法合成原位锂化V2O5 (PLVO)纳米棒,将大的、无序的颗粒转化为精细的纳米结构。这种方法避免了昂贵、耗时的烧结和碳涂层过程。由此产生的柔性,独立的PLVO电极具有优异的性能,在10C和20C下分别达到276 mAh/g和213 mAh/g。在1C下850次循环后,它保持了383 mAh/g, 99.4%的库仑效率和79%的容量保持率。在llic中,它在0.533 a /g时提供153.95 F/g的高比电容,在0.266 kW/kg时提供192.44 Wh/kg的能量密度,在13.33 kW/kg时提供62.69 Wh/kg的能量密度,在10,000次循环后保持86.46%。这些结果突出了PLVO电极的强大速率能力,耐用性以及灵活,高性能储能设备的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
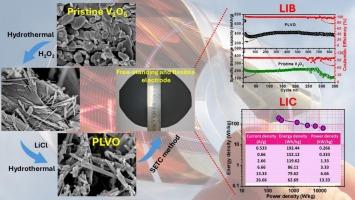
In-situ lithiated and shape-modified V2O5 nanorods as anode materials for high-performance Li-ion batteries and Li-ion capacitors
Vanadium pentoxide (V2O5) is a promising electrode material for next-generation lithium-ion batteries (LIBs) and lithium-ion capacitors (LICs), but its poor structural and electrochemical stability limits performance. This study presents a two-step hydrothermal method to synthesize in-situ lithiated V2O5 (PLVO) nanorods, transforming large, disordered particles into fine nanostructures. This method avoids the costly, time-consuming sintering and carbon coating process. The resulting flexible, free-standing PLVO electrode delivers excellent performance, achieving 276 mAh/g at 10C and 213 mAh/g at 20C. It maintains 383 mAh/g with 99.4 % coulombic efficiency and 79 % capacity retention after 850 cycles at 1C. In LICs, it provides a high specific capacitance of 153.95 F/g at 0.533 A/g, an energy density of 192.44 Wh/kg at 0.266 kW/kg, and 62.69 Wh/kg at 13.33 kW/kg, with 86.46 % retention after 10,000 cycles. These results highlight the PLVO electrode's strong rate capability, durability, and potential for flexible, high-performance energy storage devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


