槲皮素和omega-3脂肪酸负载的脂质混合纳米组件作为有前途的抗菌生物膜策略
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
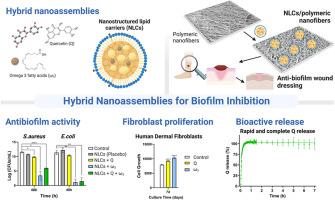
生物膜的形成通常与抗菌治疗失败和慢性感染的发展有关。因此,制定新的策略来解决伤口治疗是很重要的。纳米载体可以精确地设计,针对特定的细菌菌株,或破坏生物膜结构。这项工作旨在评估槲皮素(Q)和/或ω -3脂肪酸(ω3)负载在由纳米结构脂质载体(NLCs)和纳米纤维组成的混合纳米组件中的潜力,作为防止伤口生物膜形成的创新策略。NLCs在尺寸和电荷方面均匀,平均水动力直径小于200 nm,带负电荷。分析了NLCs上游离和负载的Q和ω3对金黄色葡萄球菌和大肠杆菌生物膜的抗菌活性。NLCs具有抑制作用,特别是那些含有ω3的。与游离化合物相比,包封的化合物也显示出更高的抗生物膜活性。NLCs中游离或负载的Q和/或ω3对细胞培养成纤维细胞无细胞毒性。7天后,NLCs,特别是含有ω3的NLCs,对细胞增殖有积极作用,可能有助于组织再生。富含nnc的纳米纤维具有多孔结构,允许渗出液、气体和营养物质的交换,同时它们的随机取向模仿皮肤的细胞外基质,帮助细胞粘附和增殖。纳米纤维具有良好的机械强度和增加的表面积,促进扩散,使Q快速和完全释放,这有利于抗生物膜活性。富含Q和ω3负载NLCs的纳米纤维可能是一种很有前途的抗生物膜治疗策略,允许这些具有抗菌活性的药物适当递送,同时防止伤口形成生物膜。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quercetin and omega-3 fatty acids-loaded lipid hybrid nanoassemblies as promising antimicrobial biofilm strategies
Biofilm formation is usually associated with antimicrobial treatment failure and the development of chronic infections. Therefore, it is important to develop new strategies to address wounds treatment. Nanocarriers can be engineered with precision, tailored to target specific bacterial strains, or disrupt biofilm architecture. This work aimed to evaluate the potential of quercetin (Q) and/or omega-3 fatty acids (ω3)-loaded in hybrid nanoassemblies composed by nanostructured lipid carriers (NLCs) and nanofibers as an innovative strategy to prevent biofilm formation on wounds. NLCs were homogeneous in terms of size and charge, with mean hydrodynamic diameter less than 200 nm and a negative charge. The antibiofilm activity of Q and ω3, both free and loaded on NLCs, on S. aureus and E. coli biofilms, was analysed. NLCs have an inhibitory effect, especially those containing ω3. The encapsulated compounds also demonstrated increased antibiofilm activity compared to free compounds. Q and/or ω3, free or loaded in NLCs, showed no cytotoxicity on cell-cultured fibroblasts. After 7 days, NLCs, especially those containing ω3, had a positive effect on cell proliferation, potentially aiding in tissue regeneration. NLC-enriched nanofibers with a porous structure allow for exchange of exudates, gases, and nutrients, while their random orientation mimics the skin's extracellular matrix, aiding cell adhesion and proliferation. The nanofibers present good mechanical strength and an increased surface area that enhances diffusion, enabling rapid and complete Q release, which is beneficial for anti-biofilm activity.
Q and ω3-loaded NLCs enriched nanofibers may be a promising antibiofilm treatment strategy, allowing for proper delivery of these agents with antimicrobial activity while preventing biofilm formation on wounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Nano
Multiple-
CiteScore
11.30
自引率
3.90%
发文量
130
审稿时长
31 days
期刊介绍:
Materials Today Nano is a multidisciplinary journal dedicated to nanoscience and nanotechnology. The journal aims to showcase the latest advances in nanoscience and provide a platform for discussing new concepts and applications. With rigorous peer review, rapid decisions, and high visibility, Materials Today Nano offers authors the opportunity to publish comprehensive articles, short communications, and reviews on a wide range of topics in nanoscience. The editors welcome comprehensive articles, short communications and reviews on topics including but not limited to:
Nanoscale synthesis and assembly
Nanoscale characterization
Nanoscale fabrication
Nanoelectronics and molecular electronics
Nanomedicine
Nanomechanics
Nanosensors
Nanophotonics
Nanocomposites
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


