GTX-11改善实验性MASH中门静脉高压、肝纤维化和窦细胞表型
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
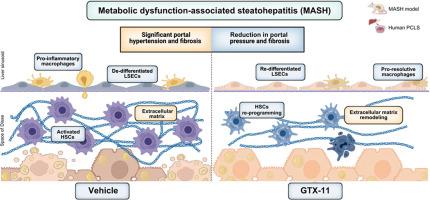
背景和目的脂肪变性肝病可导致代谢功能障碍相关脂肪性肝炎(MASH)的发展,其患病率正在迅速增加,迫切需要寻找有效的治疗方法。GTX-11是一种通过调节转化生长因子β途径介导的抗炎和抗纤维化特性的一流药物。本研究评估了GTX-11对肝脏血流动力学和肝纤维化的影响,以及其在临床前MASH模型和人类精确切割肝脏切片(hPCLS)中的潜在机制。方法雄性Wistar MASH大鼠分别给予GTX-11(1、10 mg/kg/d)或对照,试验期14 d (n = 15/组)。分析了体内系统和肝脏血流动力学、纤维化、生化参数和肝细胞表型。从人肝切除术中获得hPCLS,并与GTX-11活性代谢产物(GTX-11m) (1 μM或10 μM)或载体孵育24小时。通过RNA测序评估基因表达变化并进行基因反卷积分析(n = 6/组)。结果GTX-11治疗的smash大鼠门静脉压力与对照组相比呈剂量依赖性降低(1和10 mg/kg组分别为-8.4% p = 0.05和-11.7% p <0.01), 10 mg/kg组肝纤维化降低(-28%,p <0.01)。在细胞水平上,gtx -11处理的大鼠表现为肝星状细胞失活和内皮细胞再分化。来自hPCLS的转录组学分析显示,GTX-11m促进hsc失活,抑制促纤维化途径,以及细胞外基质重塑。基因反褶积分析证实了GTX-11m对hsc的有益作用,促进其失活和再平衡。结论本研究首次证实了GTX-11通过造血干细胞失活和内皮表型恢复对MASH门脉高压和肝纤维化的有益作用。在人肝组织中的验证促进了其作为一种可能的新治疗方法的临床评估。影响和意义代谢功能障碍相关脂肪性肝炎(MASH)影响着全球3-6%的人口,而且这一数字还在不断增加。尽管发病率很高,但目前尚无针对该疾病的具体、普遍有效和安全的治疗方法。这项研究首次证明了GTX-11通过使肝星状细胞失活和增强内皮表型来治疗与MASH相关的纤维化和门脉高压的治疗潜力。这些发现突出了GTX-11作为未来旨在逆转肝纤维化和改善MASH患者预后的治疗策略的有希望的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
GTX-11 improves portal hypertension, liver fibrosis, and sinusoidal cells phenotype in experimental MASH
Background & Aims
Steatotic liver disease can lead to the development of metabolic dysfunction-associated steatohepatitis (MASH), the prevalence of which is rapidly increasing, intensifying the need to find an effective treatment. GTX-11 is a first-in-class drug with anti-inflammatory and antifibrotic properties mediated by the modulation of the transforming growth factor beta pathway. The present study evaluated the effects of GTX-11 on hepatic hemodynamics and liver fibrosis, as well as its underlying mechanisms, in a preclinical model of MASH, and in human precision-cut liver slices (hPCLS).
Methods
Male Wistar MASH rats received GTX-11 (1 and 10 mg/kg/day) or vehicle, for 14 days (n = 15/group). In vivo systemic and hepatic hemodynamics, fibrosis, biochemical parameters, and hepatic cells phenotype were analyzed. hPCLS were obtained from human hepatic resections and incubated with the active metabolite of GTX-11 (GTX-11m) (1 μM or 10 μM) or vehicle for 24 h. Gene expression changes were evaluated by RNA sequencing and gene deconvolution analysis was performed (n = 6/group).
Results
MASH rats receiving GTX-11 showed a significant dose-dependent reduction in portal pressure compared with the vehicle (-8.4% p = 0.05 and -11.7% p <0.01 for 1 and 10 mg/kg, respectively), associated with lower hepatic fibrosis at 10 mg/kg (-28%, p <0.01). At the cellular level, GTX-11-treated rats showed hepatic stellate cells (HSCs) deactivation and endothelial cells redifferentiation. Transcriptomic analysis from hPCLS revealed that GTX-11m promoted HSCs deactivation and inhibition of pro-fibrogenic pathways, as well as extracellular matrix remodeling. Gene deconvolution analysis confirmed the beneficial effects of GTX-11m on HSCs, promoting their deactivation and rebalance.
Conclusions
This study demonstrates for the first time the beneficial effects of GTX-11 on portal hypertension and liver fibrosis in MASH by means of HSCs deactivation and endothelial phenotype restoration. Validation in human liver tissues encourages its clinical evaluation as a possible new treatment for this disease.
Impact and implications
Metabolic dysfunction-associated steatohepatitis (MASH) affects >3–6% of the world’s population, and this number continues to increase. Despite its high prevalence, there is currently no specific, generally effective, and safe treatment for the disease. This study demonstrates, for the first time, the therapeutic potential of GTX-11 in treating fibrosis and portal hypertension associated with MASH by deactivating hepatic stellate cells and enhancing endothelial phenotype. These findings highlight GTX-11 as a promising candidate for future therapeutic strategies aimed at reversing liver fibrosis and improving patient outcomes in MASH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


