高浓度l -蛋氨酸在高浓度抗体治疗中作为抗氧化和增强稳定性的有效抗氧化剂
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
用于皮下给药的高浓度单克隆抗体(mAb)制剂的开发面临着关键的稳定性挑战,特别是氧化和聚集,这损害了其疗效和安全性。虽然抗氧化剂通常被使用,但现有的研究主要集中在低浓度,而对高浓度抗氧化剂的潜力尚未充分探索。在这里,我们提出了高浓度l -蛋氨酸(L-Met,>20 mM)作为一种新型抗氧化剂的第一个系统评价,以解决这些局限性。通过加速稳定性测试和多维分析技术,我们证明了浓度超过20 mM的L-Met在减轻氧化和聚集方面优于传统抗氧化剂。与200 mM海藻糖协同作用,通过减少氧化降解和抑制蛋白质聚集进一步增强稳定性。综合生物物理分析证实无副作用,某些方面显示结构完整性、胶体稳定性和热行为得到改善。优化后的配方(25 mM L-Met +200 mM海藻糖)也显示出对光诱导降解的强大保护作用,并且广泛适用于治疗性抗体。这项工作开创了高浓度抗氧化策略,解决了单抗制剂科学的关键空白,并为稳定下一代高浓度生物制剂提供了可翻译的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
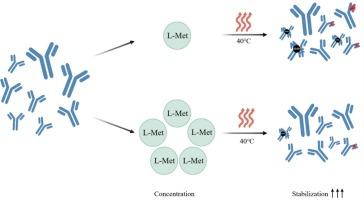
High-concentration L-methionine as a potent antioxidant for oxidation resistance and stability enhancement in high-concentration antibody therapeutics
The development of high-concentration monoclonal antibody (mAb) formulations for subcutaneous administration is faces critical stability challenges, particularly oxidation and aggregation, which compromise efficacy and safety. While antioxidants are commonly employed, existing studies predominantly focus on low concentrations, leaving the potential of high-concentration antioxidants underexplored. Here, we present the first systematic evaluation of high-concentration L-methionine (L-Met,>20 mM) as a novel antioxidant to address these limitations. Through accelerated stability testing coupled with multi-dimensional analytical techniques, we demonstrated that L-Met at concentrations exceeding 20 mM surpasses conventional antioxidants in mitigating oxidation and aggregation. Synergy with 200 mM trehalose further enhanced stability by reducing oxidative degradation and inhibiting protein aggregation. Comprehensive biophysical analyses confirmed no adverse effects, with some aspects showing improved outcomes in structural integrity, colloidal stability, and thermal behavior. The optimized formulation (25 mM L-Met +200 mM trehalose) also exhibited robust protection against light-induced degradation and broad applicability across therapeutic antibodies. This work pioneers a high-concentration antioxidant strategy, addressing a critical gap in mAb formulation science and offering a translatable solution for stabilizing next-generation high-concentration biologics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


