促炎表型在确定和调节非综合征性听力损失的遗传风险方面至关重要
IF 2.2
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
摘要
非综合征性听力损失(NSHL)是一种常见的感觉障碍,具有多因素的起源,涉及遗传和环境因素。其遗传基础表现出显著的变异和不完全外显性。环境因素,特别是TORCH感染和无菌炎症,可能通过触发炎症级联反应而导致NSHL。细胞因子,在免疫调节中至关重要的分泌蛋白,在介导这些炎症反应中起着核心作用。本研究探讨细胞因子基因的遗传变异是否与NSHL易感性有关。在说马拉雅拉姆语的德拉威人群体中进行了一项病例对照遗传关联研究,重点关注促炎性和抗炎性细胞因子基因变异。研究结果首次揭示了NSHL与促炎细胞因子IL1A、IFNG、IL3和IL12B变异以及抗炎细胞因子IL4之间的强烈关联。这些风险变异与促炎活性增加和抗炎反应减少有关。相互作用组分析显示,细胞因子基因IL6、IL1B和TNF与关键候选基因如GSDME、DIABLO和GJA1之间存在相互作用。结果提示了另一种NSHL发病机制,其中环境影响可能通过细胞因子基因变异影响基因表达,可能通过“金发姑娘效应”。这项研究强调了遗传和环境因素在NSHL发展中的复杂相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
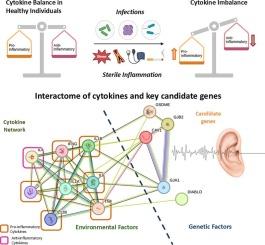
Proinflammatory phenotype can be critical in defining and modulating the genetic risk of non-syndromic hearing loss
Non-syndromic hearing loss (NSHL) is a common sensory disorder with a multifactorial origin, involving both genetic and environmental components. Its genetic basis shows significant variability and incomplete penetrance across populations. Environmental factors, especially TORCH infections and sterile inflammation, may contribute to NSHL by triggering inflammatory cascades. Cytokines, secreted proteins crucial in immune regulation, play a central role in mediating these inflammatory responses. This study investigates whether genetic variants in cytokine genes contribute to NSHL susceptibility. A case-control genetic association study was conducted in the Malayalam-speaking Dravidian population, focusing on both pro- and anti-inflammatory cytokine gene variants. The findings reveal, for the first time, a strong association between NSHL and variants in pro-inflammatory cytokines IL1A, IFNG, IL3, and IL12B, along with the anti-inflammatory cytokine IL4. These risk variants were associated with increased pro-inflammatory activity and reduced anti-inflammatory responses. Interactome analysis showed interactions among cytokine genes IL6, IL1B, and TNF with key candidate genes such as GSDME, DIABLO, and GJA1. The results suggest an alternative NSHL pathogenesis mechanism, where environmental influences may act through cytokine gene variants to affect gene expression, possibly via a “Goldilocks effect.” This study underscores the complex interplay of genetic and environmental factors in NSHL development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Human Immunology
医学-免疫学
CiteScore
5.40
自引率
7.40%
发文量
107
审稿时长
12 days
期刊介绍:
The journal''s scope includes understanding the genetic and functional mechanisms that distinguish human individuals in their immune responses to allografts, pregnancy, infections or vaccines as well as the immune responses that lead to autoimmunity, allergy or drug hypersensitivity. It also includes examining the distribution of the genes controlling these responses in populations.
Research areas include:
Studies of the genetics, genomics, polymorphism, evolution, and population distribution of immune-related genes
Studies of the expression, structure and function of the products of immune-related genes
Immunogenetics of susceptibility to infectious and autoimmune disease, and allergy
The role of the immune-related genes in hematopoietic stem cell, solid organ, and vascularized composite allograft transplant
Histocompatibility studies including alloantibodies, epitope definition, and T cell alloreactivity
Studies of immunologic tolerance and pregnancy
T cell, B cell, NK and regulatory cell functions, particularly related to subjects within the journal''s scope
Pharmacogenomics and vaccine development in the context of immune-related genes
Human Immunology considers immune-related genes to include those encoding classical and non-classical HLA, KIR, MIC, minor histocompatibility antigens (mHAg), immunoglobulins, TCR, BCR, proteins involved in antigen processing and presentation, complement, Fc receptors, chemokines and cytokines. Other immune-related genes may be considered.
Human Immunology is also interested in bioinformatics of immune-related genes and organizational topics impacting laboratory processes, organ allocation, clinical strategies, and registries related to autoimmunity and transplantation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


