双模成像伴热推动的协同癌症治疗的可激活伴治疗
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
伴随诊断(CDx)在癌症治疗的精准医学中起着关键作用。然而,传统的CDx常常由于无法提供癌症进展和治疗反应的实时监测而受到限制。在此,我们开发了一种基于双模成像的伴随治疗(CTx)纳米平台(LET-Cl@GOx),它集成了可激活的光声(PA)和荧光(FL)成像,以提高诊断准确性和实时治疗反馈,同时展示了级联放大的光热/饥饿协同治疗。LET-Cl@GOx是由葡萄糖氧化酶(GOx)与ph可激活的近红外(NIR)染料(LET-Cl)组装而成,能够在酸性肿瘤微环境(TME)中开启PA/FL成像。PA/FL成像信号的动态变化提供了TME酸化的实时反馈,从而能够精确监测GOx催化过程和光热治疗(PTT)干预的精确定时。此外,gox介导的肿瘤饥饿降低了三磷酸腺苷(ATP)水平,导致热休克蛋白表达减少,从而增强了对PTT的敏感性。同时,光热效应相互增强了GOx的催化活性,形成了一个正反馈放大的三重闭环系统。这种多尺度增强的协同疗法通过Caspase-3/gasdermin E信号通路触发强大的焦亡,在体内显示出显著的肿瘤治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
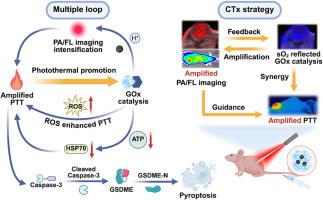
Activatable companion theranostics for dual-modality imaging-escorted pyroptosis-propelled synergistic cancer therapy
Companion diagnostics (CDx) plays a pivotal role in precision medicine for cancer treatment. However, conventional CDx are often limited by their inability to provide real-time monitoring of cancer progression and therapeutic responses. Herein, we develop a dual-modality imaging-based companion theranostic (CTx) nanoplatform (LET-Cl@GOx), which integrates activatable photoacoustic (PA) and fluorescence (FL) imaging to enable the enhanced diagnostic accuracy and real-time therapeutic feedback, while demonstrating cascade-amplified photothermal/starvation synergistic therapy. The LET-Cl@GOx is designed by the assembly of glucose oxidase (GOx) with a pH-activatable near-infrared (NIR) dye (LET-Cl), enabling the turn-on of PA/FL imaging within the acidic tumor microenvironment (TME). The dynamic alterations of PA/FL imaging signals provide real-time feedback on TME acidification, enabling accurate monitoring of GOx catalysis progression and precision timing of photothermal therapy (PTT) intervention. Furthermore, the GOx-mediated tumor starvation reduces adenosine triphosphate (ATP) levels, leading to the diminished heat shock protein expression and consequently enhanced the sensitivity to PTT. Concurrently, the photothermal effect reciprocally enhances the catalytic activity of GOx, establishing a triple closed-loop system with positive feedback amplification. This multiscale-augmented synergistic therapy triggers robust pyroptosis via the Caspase-3/gasdermin E signaling pathway, demonstrating remarkable therapeutic efficacy of tumors in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


