使用三唑基抑制剂增强金属合金耐腐蚀性和化学耐久性的表面驱动相互作用:合成、表征和计算观点
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究以两种新合成的杂环化合物5-戊基磺酰-3-苯基-1,2,4-三唑(PTri-C5H11)和5-戊基磺酰-3-十一烷基-1,2,4-三唑(UTri-C5H11)为研究对象,探讨了环境友好型缓蚀剂的潜力。在常温条件下通过固-液相转移催化合成了这些新型的1,2,4-三唑-5-硫酮衍生物。以碘戊烷为烷基化剂,对前体三唑-硫酮化合物进行烷基化反应。通过光谱技术确定了合成化合物的结构特征,并通过实验和理论相结合的方法对其缓蚀性能进行了评价。结果表明,抑制效率依次为:PTri-C5H11 >; UTri-C5H11,这是由于苯环的给受体能力和共轭π电子与碳钢(CS)表面形成配位配合物所致。在浓度为10−3 M时,化合物对PTri-C5H11和UTri-C5H11的缓蚀效率分别为92.98%和91.96%。为了获得分子水平的认识,采用密度泛函理论(DFT)和密度泛函紧密结合(DFTB)计算来探索所研究抑制剂的量子化学性质、吸附特性和界面相互作用。表面吸附研究进一步揭示了抑制剂与CS表面之间形成强共价键,电子密度分析证实了这一点。理论模拟表明,这两种抑制剂都可以通过C、S和N原子在平行方向上吸附,从而最大限度地提高表面覆盖率。在这个方向上,两种抑制剂的C-Fe, S-Fe和N-Fe键距离从1.98 Å到2.40 Å不等。因此,实验和理论结果是一致的,证实了杂原子(1,2,4-三唑-5-硫酮基团)和苯环(对于PTri-C5H11)是CS表面的主要吸附位点。总的来说,这些发现表明了这些1,2,4-三唑衍生物在酸性环境中作为有效的CS缓蚀剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
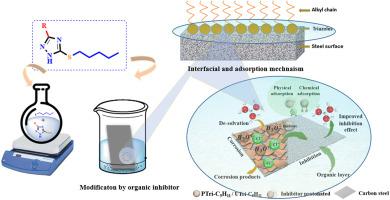
Surface-driven interactions for enhanced corrosion resistance and chemical durability of metal alloys using triazole-based inhibitors: Synthesis, characterization, and computational perspectives
This study investigates the potential of environmentally friendly corrosion inhibitors by focusing on two newly synthesized heterocyclic compounds, namely 5-pentylsulfanyl-3-phenyl-1,2,4-triazole (PTri-C5H11) and 5-pentylsulfanyl-3-undecyl-1,2,4-triazole (UTri-C5H11). These novel 1,2,4-triazole-5-thione derivatives were synthesized via solid-liquid phase transfer catalysis at ambient temperature conditions. The synthesis involved the alkylation of precursor triazole-thione compounds using iodopentane as the alkylating agent. The structural features of the synthesized compounds were confirmed by spectroscopic techniques, while their corrosion inhibition performance was evaluated through a combination of experimental and theoretical approaches. Results indicated that the inhibition efficiency followed the order: PTri-C5H11 > UTri-C5H11, attributed to the donor-acceptor capability of the benzene ring and conjugated π-electrons forming coordination complexes with the carbon steel (CS) surface. At a concentration of 10−3 M, the tested compounds significantly enhanced corrosion resistance, achieving inhibition efficiencies of 92.98 % and 91.96 % for PTri-C5H11 and UTri-C5H11, respectively. To gain molecular-level insights, density functional theory (DFT) and density functional tight binding (DFTB) calculations were employed to explore the quantum chemical properties, adsorption characteristics, and interfacial interactions of the studied inhibitors Surface adsorption studies further revealed the formation of strong covalent bonds between the inhibitors and the CS surface, confirmed by the electron density analysis. Theoretical modeling showed that both inhibitors could adsorb in a parallel orientation via C, S, and N atoms, which maximizes the surface coverage. In this orientation, C–Fe, S–Fe, and N–Fe bond distances ranged, for both inhibitors, from 1.98 Å to 2.40 Å. Thus, experimental and theoretical findings are consistent, confirming that heteroatoms (1,2,4-triazole-5-thione group) and benzene ring (for PTri-C5H11) serve as primary adsorption sites on the CS surface. Overall, these findings demonstrate the promising potential of these 1,2,4-triazole derivatives as effective corrosion inhibitors for CS in acidic environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


