泪腺核心昼夜节律基因Nr1d2的下调通过损害脂质代谢参与术后干眼病
IF 5.6
1区 医学
Q1 OPHTHALMOLOGY
引用次数: 0
摘要
目的:干眼病是屈光手术后常见的并发症,其发病机制尚不完全清楚。鉴于昼夜节律紧密调控泪液分泌,本研究旨在探讨角膜屈光手术后泪腺的昼夜节律变化及其机制。方法:对C57/BL6小鼠进行角膜上皮磨损和间质切断。采用酚红线和荧光素染色评价DED。RNA测序鉴定出与昼夜节律相关的差异表达基因(DEGs),并通过RT-qPCR和Western blotting验证。采用免疫荧光、TUNEL染色、非靶向代谢组学、Oil-Red-O染色和透射电镜分析泪腺的变化。临床应用OSDI问卷对867例术后患者和705例对照患者进行DED症状评估。结果:角膜上皮磨损和间质损伤引起DED症状和泪液功能障碍,包括泪液量减少、眼表炎症和泪液病理生理改变。与对照组相比,关键的昼夜节律基因Nr1d2在泪腺中显著下调。SR9011激活Nr1d2恢复泪腺功能,缓解DED症状。此外,Nr1d2的下调显著损害了泪腺的脂质代谢和线粒体功能,SR9011逆转了这一变化。临床上,手术组DED的发生率明显较高,且症状呈现昼夜变化,在清晨和傍晚达到高峰。结论:昼夜节律的破坏,特别是Nr1d2的下调,有助于术后DED。靶向Nr1d2可能为恢复泪腺功能和缓解DED提供新的治疗途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
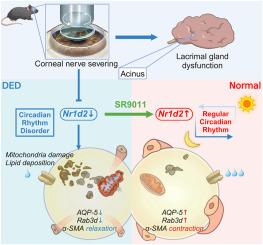
Downregulation of the core circadian gene Nr1d2 in the lacrimal gland contributes to postoperative dry eye disease by impairing lipid metabolism
Purpose
Dry eye disease (DED) is a prevalent complication after refractive surgery, and its underlying mechanisms are not fully understood. Given that circadian rhythm tightly regulates tear secretion, this study aims to investigate the circadian changes in the lacrimal gland after corneal refractive surgery and the associated mechanisms.
Methods
C57/BL6 mice underwent corneal epithelial abrasion and stromal severing. DED was assessed using phenol red thread and fluorescein staining. RNA sequencing identified differentially expressed genes (DEGs) related to circadian rhythm, validated by RT-qPCR and Western blotting. Immunofluorescence, TUNEL staining, untargeted metabolomics, Oil-Red-O staining, and TEM were performed to analyze the lacrimal gland changes. Clinically, DED symptoms were evaluated using the OSDI questionnaire in 867 post-surgical patients and 705 controls.
Results
Corneal epithelial abrasion and stromal severing induced DED symptoms and lacrimal dysfunction, including reduced tear volume, ocular surface inflammation, and lacrimal pathophysiological changes. Nr1d2, the key circadian rhythm gene, was significantly down-regulated in the lacrimal gland compared to the control. Activation of Nr1d2 with SR9011 restored the lacrimal gland function and alleviated DED symptoms. Furthermore, the down-regulation of Nr1d2 significantly impaired lipid metabolism and mitochondrial function in the lacrimal gland, which SR9011 reversed. Clinically, the incidence of DED was markedly higher in the surgical cohort, with symptom occurrence exhibiting diurnal variation and peaking in the early morning and late evening.
Conclusions
Disruption of circadian rhythms, specifically Nr1d2 downregulation, contributes to post-surgical DED. Targeting Nr1d2 may offer a novel therapeutic approach to restore lacrimal gland function and alleviate DED.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Ocular Surface
医学-眼科学
CiteScore
11.60
自引率
14.10%
发文量
97
审稿时长
39 days
期刊介绍:
The Ocular Surface, a quarterly, a peer-reviewed journal, is an authoritative resource that integrates and interprets major findings in diverse fields related to the ocular surface, including ophthalmology, optometry, genetics, molecular biology, pharmacology, immunology, infectious disease, and epidemiology. Its critical review articles cover the most current knowledge on medical and surgical management of ocular surface pathology, new understandings of ocular surface physiology, the meaning of recent discoveries on how the ocular surface responds to injury and disease, and updates on drug and device development. The journal also publishes select original research reports and articles describing cutting-edge techniques and technology in the field.
Benefits to authors
We also provide many author benefits, such as free PDFs, a liberal copyright policy, special discounts on Elsevier publications and much more. Please click here for more information on our author services.
Please see our Guide for Authors for information on article submission. If you require any further information or help, please visit our Support Center
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


