类胡萝卜素结合视紫红质,在海洋拟杆菌群中作为光循环加速色素
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
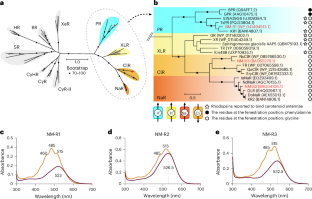
微生物视紫红质是广泛存在于海洋微生物体内的光感受器蛋白,具有利用光能和支持海洋生态系统的作用。虽然视网膜通常是微生物紫红质中唯一的发色团,但海洋中富含质子泵紫红质的一些蛋白紫红质使用类胡萝卜素天线将光能传输到视网膜。然而,类胡萝卜素增强视紫红质功能的机制尚不清楚。在这里,我们利用海洋拟杆菌属分离物Nonlabens marinus S1-08T,重建了与类胡萝卜素黏液的视紫红质复合物,并检测了变形视紫红质和氯离子泵送视紫红质向视网膜的能量传递。类胡萝卜素结合促进了光收集,加速了光循环,从而提高了变形紫质对光的利用效率。低温电镜结构分析进一步揭示了类胡萝卜素-视紫红质复合物的分子结构。结合类胡萝卜素的能力在海洋优势门拟杆菌门的视紫红质中是保守的,它在光区广泛转录。这些发现揭示了类胡萝卜素如何增强海洋拟杆菌群的视紫红质功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Carotenoids bind rhodopsins and act as photocycle-accelerating pigments in marine Bacteroidota
Microbial rhodopsins are photoreceptor proteins widely distributed in marine microorganisms that harness light energy and support marine ecosystems. While retinal is typically the sole chromophore in microbial rhodopsins, some proteorhodopsins, which are proton-pumping rhodopsins abundant in the ocean, use carotenoid antennae to transfer light energy to retinal. However, the mechanism by which carotenoids enhance rhodopsin functions remains unclear. Here, using the marine Bacteroidota isolate Nonlabens marinus S1-08T, we reconstituted complexes of rhodopsins with the carotenoid myxol and detected energy transfer to retinal in both proteorhodopsin and chloride ion-pumping rhodopsin. Carotenoid binding facilitated light harvesting and accelerated the photocycle, thereby improving the light utilization efficiency of proteorhodopsin. Cryogenic electron microscopy structural analysis further revealed the molecular architecture of the carotenoid–rhodopsin complexes. The ability to bind carotenoids is conserved in rhodopsins of the marine-dominant phylum Bacteroidota, which are widely transcribed in the photic zone. These findings reveal how carotenoids enhance rhodopsin functions in marine Bacteroidota. In marine bacteria, carotenoids enhance rhodopsin function by acting as light-harvesting antennae and photocycle-accelerating pigments, a dual mechanism that enhances light energy capture and expands the known strategies of microbial phototrophy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


