木质素在Ni/CeO2上通过氢转移选择性加氢脱氧成环己醇
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在无氢条件下,由可再生木质素生产高价值环己醇具有很高的吸引力,但众所周知具有挑战性。在这里,我们报道了一种有效的策略,将各种木质素衍生的酚类单体和木质素油转化为环己醇,使用Ni/CeO2催化剂,异丙醇作为氢供体。这种方法从2-甲氧基-4-丙基苯酚中获得了令人印象印象的90.1 %的产率,证明了芳香环的选择性氢化和Ar-OCH3键的裂解,同时保留了所需的羟基功能。机理研究表明,Ni和CeO2之间的相互作用促进了Ni的还原和氧空位的产生。此外,Ni和CeO2之间的电荷转移促进了Niδ+的形成,促进了芳环的吸附和活化。值得注意的是,Ni/CeO2催化剂表现出优异的稳定性和广泛的适用性,从真正的木质素油中获得66.6%的环己醇收率。本研究提出了一种通过催化氢转移反应将可再生木质素高选择性转化为环己醇的稳健策略,为木质素的可持续利用铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。

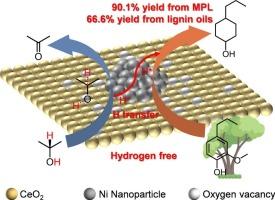
Selective hydrodeoxygenation of lignins into cyclohexanols over Ni/CeO2 via hydrogen transfer
The production of high-value cyclohexanols from renewable lignins under hydrogen-free conditions is highly attractive but notoriously challenging. Here, we report an efficient strategy for converting various lignin-derived phenolic monomers and lignin oils into cyclohexanols using a Ni/CeO2 catalyst with isopropanol as the hydrogen donor. This approach achieves an impressive yield of 90.1 % from 2-methoxy-4-propylphenol, demonstrating selective hydrogenation of aromatic rings and cleavage of Ar-OCH3 bonds while retaining the desired hydroxyl functionalities. Mechanistic investigations reveal that the interplay between Ni and CeO2 enhances the reduction of Ni species and the creation of oxygen vacancies. Additionally, the charge transfer between Ni and CeO2 facilitates the formation of Niδ+, which promotes the adsorption and activation of aromatic rings. Notably, the Ni/CeO2 catalyst exhibits excellent stability and broad applicability, delivering a cyclohexanol yield of 66.6% from real lignin oil. This work presents a robust strategy for the high-selectivity conversion of renewable lignin into cyclohexanols via catalytic hydrogen transfer reactions, paving the way for sustainable lignin utilization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


