骨转移中的成骨细胞:肿瘤微环境和治疗靶点的关键参与者。
IF 9.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Reviews on cancer
Pub Date : 2025-08-29
DOI:10.1016/j.bbcan.2025.189435
引用次数: 0
摘要
成骨细胞因其在骨形成和矿物质代谢中的作用而得到认可,虽然尚未得到充分的研究,但它们正在成为骨肿瘤微环境(TME)中的关键调节因子。尽管越来越多的证据表明它们的参与,但它们对转移进展的确切贡献仍未得到充分认识。最近的研究表明,成骨细胞通过动态的、阶段特异性的相互作用来协调转移。在早期定植中,它们可能通过CXCL12/CXCR4信号吸引肿瘤细胞并重塑转移生态位。在休眠期间,成骨细胞衍生因子如LIF、TGFβ2和BMP7,以及粘附分子如N-cadherin,促进治疗抵抗。随后,成骨细胞可以通过代谢偶联(如Ca2+转移)和mTOR通路激活来驱动转移性生长。除了这些对肿瘤细胞的直接作用外,成骨细胞还通过与破骨细胞和免疫细胞相互作用来调节TME,抑制CD8+ T/NK细胞活性,同时扭曲巨噬细胞极化以促进免疫逃避。肿瘤来源的信号包括PTHrP、bmp和ET-1,进一步将成骨细胞重编程为肿瘤支持表型。RANKL抑制、CXCL12通路阻断、TGF-β超家族拮抗、成骨细胞靶向免疫治疗等治疗方法为临床干预提供了有希望的方向。认识到成骨细胞在骨转移中的核心作用可能为骨靶向癌症治疗提供新的领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
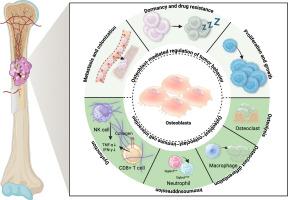
Osteoblasts in bone metastasis: Key players in the tumor microenvironment and therapeutic targets
Osteoblasts, recognized for their role in bone formation and mineral metabolism, are emerging as pivotal, although underexplored, regulators within the bone tumor microenvironment (TME). Despite increasing evidence of their involvement, their precise contributions to metastatic progression remain underappreciated. Recent studies reveal that osteoblasts orchestrate metastasis through dynamic, stage-specific interactions. In early colonization, they may attract tumor cells via CXCL12/CXCR4 signaling and remodel the metastatic niche. During dormancy, osteoblast-derived factors such as LIF, TGFβ2, and BMP7, along with adhesion molecules such as N-cadherin, promote therapy resistance. Subsequently, osteoblasts can drive metastatic outgrowth through metabolic coupling (e.g., Ca2+ transfer) and mTOR pathway activation. Beyond these direct effects on tumor cells, osteoblasts modulate the TME by interacting with osteoclasts and immune cells, suppressing CD8+ T/NK cell activity while skewing macrophage polarization to promote immune evasion. Tumor-derived signals including PTHrP, BMPs, and ET-1, further reprogram osteoblasts into a tumor-supportive phenotype. Therapeutic approaches, such as RANKL inhibition, CXCL12 pathway blockade, TGF-β superfamily antagonism, and osteoblast-targeted immunotherapies, offer promising directions for clinical intervention. Recognizing osteoblasts as central players in bone metastasis may provide new frontiers in bone-targeted cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Reviews on cancer
医学-生化与分子生物学
CiteScore
17.20
自引率
0.00%
发文量
138
审稿时长
33 days
期刊介绍:
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer encompasses the entirety of cancer biology and biochemistry, emphasizing oncogenes and tumor suppressor genes, growth-related cell cycle control signaling, carcinogenesis mechanisms, cell transformation, immunologic control mechanisms, genetics of human (mammalian) cancer, control of cell proliferation, genetic and molecular control of organismic development, rational anti-tumor drug design. It publishes mini-reviews and full reviews.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


