研究骨骼肌在多轴拉伸和压缩条件下结构-功能机制的双相微观结构模型。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨骼肌的被动材料特性是正确传递力的关键,肌肉微观结构的改变会对整个组织功能产生有害影响。然而,据作者所知,目前还不可能通过微结构测量来预测骨骼肌的被动材料特性,如titin异构体类型和/或细胞外基质胶原蛋白含量、类型或组织。本工作的目标是:1)建立多轴载荷条件下被动骨骼肌组织长度尺度的实验数据集;2)建立骨骼肌双相微观结构模型;3)校准、验证和实现该模型。进行了平面双轴和半密闭压缩实验,并测量了肌内压力。利用GIBBON工具箱建立了基于Voronoi-like结构的微观结构有限元模型,并采用多域双相、各向异性、超粘弹性本构方法对肌肉纤维和细胞外基质进行建模。在对实验数据进行模型校准和验证后(均方根误差为0.5% - 21%),参数化研究表明,肌纤维和细胞外基质的拉伸性能最大程度地影响了各向异性的拉伸应力-拉伸行为,而渗透性则极大地影响了压应力-拉伸行为。一些参数,如细胞外基质体积分数和肌纤维体积模量,影响拉伸和压缩应力-拉伸行为。未来将该模型扩展到特定损伤条件或模拟整个肌肉的工作将对该领域有益。意义声明:骨骼肌是人体运动的主要驱动力。为了更好地了解健康和受损肌肉的功能如何预防肌肉相关损伤,我们可以使用计算建模。在这项研究中,我们开发了一种新的肌肉组织微观结构模型,其中包含液体,这在肌肉组织的计算机模型中通常被忽略。总的来说,这项工作表明,不同组织成分之间的相互作用,特别是肌肉细胞、细胞外基质和液体,可以共同促进肌肉组织的机械行为。我们的研究结果可以用来建立更好的肌肉组织计算机模型,并研究特定的机制——如肌肉组织中的液体流动——作为预防肌肉损伤(如压疮)的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
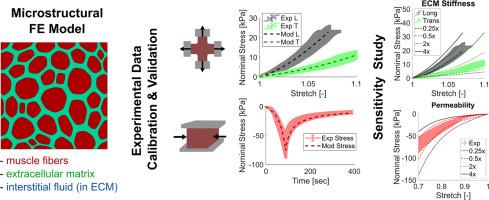
A biphasic microstructural model of skeletal muscle to study structure-function mechanisms under multiaxial tensile and compressive conditions
The passive material properties of skeletal muscle are key to proper force transmission, and changes to muscle microstructure can have deleterious effects on whole tissue function. However, to the best of the authors’ knowledge, it is not currently possible to predict the passive material properties of skeletal muscle with microstructural measurements such as titin isoform type and/or extracellular matrix collagen content, type, or organization. The goals of this work were to 1) develop an experimental dataset at the tissue length scale of passive skeletal muscle under multiaxial loading conditions, 2) develop a biphasic microstructural model of skeletal muscle, and 3) calibrate, validate, and implement such a model. Experimental planar biaxial and semi-confined compression experiments, with intramuscular pressure measurements, were conducted. A microstructural finite element model was developed using the GIBBON toolbox based on a Voronoi-like structure and a multi-domain biphasic, anisotropic, hyper-viscoelastic constitutive approach was used to model muscle fibers and the extracellular matrix. Following model calibration and validation to experimental data (with root mean square error values ranging from 0.5 % - 21 %), a parametric study suggested that tensile properties of the muscle fibers and extracellular matrix affected anisotropic tensile stress-stretch behavior to the greatest extent, while permeability greatly affected compressive stress-stretch behavior. Some parameters, such as extracellular matrix volume fraction and muscle fiber bulk modulus, affected both tensile and compressive stress-stretch behavior. Future work to expand this model into specific impairment conditions or to simulations of whole muscle would be a benefit to the field.
Statement of significance
Skeletal muscle is the primary driver of human locomotion. To better understand how healthy and impaired muscle functions towards the prevention of muscle-related impairments, we can use computational modeling. In this study, we developed a new model of the microstructure of muscle tissue that incorporates fluid, which is typically neglected in computer models of muscle tissue. Collectively, the work suggests that interactions between different tissue components, specifically muscle cells, the extracellular matrix, and fluid can collectively contribute to the mechanical behavior of muscle tissue. Our results can be used to build better computer models of muscle tissue and study specific mechanisms – such as fluid flow in muscle tissue – as ways to prevent muscle injuries such as a pressure ulcer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


