抗衰老生物材料改善骨关节炎治疗的进展。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨关节炎(OA)是一种与衰老密切相关的退行性关节疾病,目前的治疗主要局限于症状缓解,不能逆转病理进展。越来越多的证据表明,衰老细胞的积累是OA发病机制的核心驱动因素。本文系统总结了用于OA治疗的抗衰老生物材料的最新进展,强调了它们通过改善药物靶向性、延长药物释放动力学和提高生物利用度来克服传统方法局限性的潜力。我们根据其抗衰老机制和递送平台对这些生物材料进行了分类,包括聚合纳米颗粒(NPs)、纳米酶、脂质纳米颗粒(LNPs)、细胞外囊泡(EVs)、水凝胶和复合水凝胶。这些系统通过多种分子机制,如调节线粒体功能障碍、炎症信号、DNA损伤、机械过载和自噬,显示出选择性消除衰老细胞的功效。此外,本文提出了整合细胞重编程、细胞器靶向治疗、人工智能(AI)指导的抗衰老材料设计和干细胞/类器官技术的创新策略,以解决药物递送障碍和衰老微环境异质性等挑战。尽管这些生物材料在安全性、可扩展性和临床转化的监管批准方面仍需进一步验证,但抗衰老生物材料代表了OA的多维和可持续治疗选择,并有望通过针对组织变性的根本原因重塑退行性关节疾病的治疗前景。意义声明:骨关节炎(OA)是一种与年龄相关的关节疾病,目前的治疗方法仅提供症状缓解而不能停止进展。越来越多的证据表明,衰老细胞驱动OA的发展。本文综述了近年来抗衰老生物材料的研究进展,包括纳米颗粒、纳米酶、脂质载体、细胞外囊泡和水凝胶。这些系统通过调节线粒体功能障碍、炎症和DNA损伤等机制,改善药物靶向性,延长释放时间,提高生物利用度,同时选择性地消除衰老细胞。我们还重点介绍了整合细胞重编程、细胞器靶向治疗、人工智能引导设计和干细胞技术的创新方法。虽然需要进一步的验证,抗衰老生物材料提供了可持续的策略,从根本上针对OA,重塑未来退行性关节疾病的治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
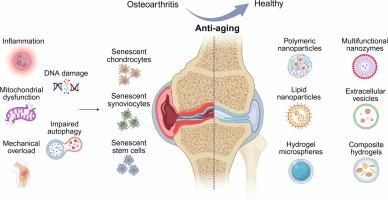
Progress in antisenescence biomaterials for improved osteoarthritis therapy
Osteoarthritis (OA) is a degenerative joint disease closely associated with aging for which current treatments are limited primarily to symptomatic relief and fail to reverse pathological progression. A growing body of evidence indicates that the accumulation of senescent cells is a central driver of OA pathogenesis. This review systematically summarizes the latest advancements in antisenescence biomaterials for OA therapy, emphasizing their potential to overcome the limitations of conventional approaches by improving drug targeting, prolonging drug release kinetics, and increasing bioavailability. We categorize these biomaterials on the basis of their antisenescence mechanism and delivery platform, including polymeric nanoparticles (NPs), nanozymes, lipid nanoparticles (LNPs), extracellular vesicles (EVs), hydrogels, and composite hydrogels. These systems have demonstrated efficacy in selectively eliminating senescent cells through various molecular mechanisms such as modulating mitochondrial dysfunction, inflammatory signaling, DNA damage, mechanical overload and autophagy. Furthermore, this review proposes innovative strategies that integrate cellular reprogramming, organelle-targeted therapy, the artificial intelligence (AI)-guided design of antisenescence materials, and stem cell/organoid technologies to address challenges such as barriers to drug delivery and the heterogeneity of the aging microenvironment. Although these biomaterials still require further validation regarding safety, scalability, and regulatory approval for their clinical translation, antisenescence biomaterials represent multidimensional and sustainable therapeutic options for OA and hold promise for reshaping the therapeutic landscape of degenerative joint diseases by targeting the root causes of tissue degeneration.
Statement of significance
Osteoarthritis (OA) is an age-related joint disease for which current therapies provide only symptom relief without halting progression. Increasing evidence shows that senescent cells drive OA development. This review summarizes recent advances in antisenescence biomaterials, including nanoparticles, nanozymes, lipid carriers, extracellular vesicles, and hydrogels. These systems improve drug targeting, prolong release, and enhance bioavailability while selectively eliminating senescent cells through mechanisms such as regulating mitochondrial dysfunction, inflammation, and DNA damage. We also highlight innovative approaches integrating cellular reprogramming, organelle-targeted therapy, AI-guided design, and stem cell technologies. Although further validation is required, antisenescence biomaterials offer sustainable strategies that target OA at its root, reshaping future treatment of degenerative joint diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


