一种内窥镜下可输送的水凝胶干粉,用于封堵和修复胃穿孔。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
胃穿孔(GP)以胃壁全层损伤为特征,是一种严重且可能危及生命的胃肠道疾病。然而,目前的治疗方法,包括手术缝合和内镜闭合,都存在局限性,实现穿孔的完全密封和良好的愈合仍然是一个巨大的挑战和迫切的临床需求。在这里,我们报道了一种用于GP微创治疗的水凝胶干粉(PPCL@Mg),它可以通过内窥镜喷洒到目标穿孔伤口,并迅速吸收界面水并自发形成水凝胶。该水凝胶具有较强的黏附性、抗胃液性、良好的消肿性、生物相容性、抗氧化、抗炎、止血等特性,可形成GP即时、坚固、无需缝合的密封。体内大鼠GP模型结果表明,PPCL@Mg水凝胶通过促进细胞增殖和血管生成,调节炎症和氧化应激,有效封闭穿孔,加速愈合,治疗效果优于手术缝合。此外,通过大动物猪GP模型进一步验证了PPCL@Mg粉末的修复功效,揭示了其比钛夹更优越的密封修复功效。转录组测序和蛋白质组学分析进一步证实了其显著的治疗效果,并阐明了其促进穿孔愈合的机制。提出的PPCL@Mg水凝胶干粉为GP修复提供了有前途的治疗方法,并展示了显著的临床转化潜力。重要意义:胃穿孔(GP)作为最严重的胃肠道急症之一,对临床提出了重大挑战,迫切需要可靠的穿孔密封和愈合。在这项工作中,我们介绍PPCL@Mg,一种用于微创GP治疗的水凝胶干粉。通过内窥镜喷射,它能迅速吸收水分并自发形成粘连的水凝胶。这种水凝胶具有很强的粘附性,抗胃液,抗肿胀,生物相容性,抗氧化,抗炎和止血特性,可以实现即时,坚固,无缝合线的GP密封。体内啮齿动物和猪模型证明PPCL@Mg水凝胶在封闭胃穿孔和加速愈合方面的功效。这种干粉系统为GP修复提供了一种有前途的策略,具有显著的转化潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
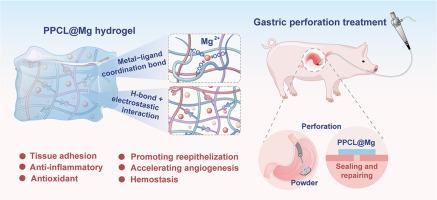
An endoscopy deliverable hydrogel dry powder for sealing and repairing gastric perforation
Gastric perforation (GP) is characterized by full-thickness injury of the stomach wall, a severe and potentially life-threatening gastrointestinal disease. However, current treatment, including surgical sutures and endoscopic closure, faces limitations, achieving complete sealing of the perforation and favorable healing remains a great challenge and an acute clinical demand. Here, we report a hydrogel dry powder (PPCL@Mg) for the minimally invasive treatment of GP, which can be delivered to target perforation wounds by spraying via an endoscope, and rapidly absorbing interfacial water and spontaneously forming a hydrogel. This hydrogel was designed with strong adhesion, gastric fluid resistance, good anti-swelling behavior, biocompatibility, anti-oxidation, anti-inflammatory and hemostatic properties, which forms instant, robust and sutureless sealing of GP. In vivo rat GP model results indicated that PPCL@Mg hydrogel effectively sealed the perforation and accelerated healing by promoting cell proliferation and angiogenesis, and regulating inflammation and oxidative stress, and the therapeutic effect surpassed that of surgical suture. Furthermore, the large animal pig GP model further validated the repair efficacy of the PPCL@Mg powder and revealed its superior sealing and repair efficacy compared to titanium clips. Transcriptome sequencing and proteomics analysis further verified the significant therapeutic outcomes and elucidated its mechanism in promoting perforation healing. The proposed PPCL@Mg hydrogel dry powder provides a promising therapeutic approach for GP repair and demonstrates significant clinical translational potential.
Statement of Significance
As one of the most severe gastrointestinal emergencies, gastric perforation (GP) poses a significant clinical challenge, where reliable perforation sealing and healing are urgently needed. In this work, we introduce PPCL@Mg, a hydrogel dry powder for minimally invasive GP treatment. Delivered endoscopically via spraying, it rapidly absorbs water and spontaneously forms an adhesive hydrogel. Engineered with strong adhesion, gastric-fluid resistance, anti-swelling, biocompatibility, anti-oxidation, anti-inflammatory and hemostatic properties, this hydrogel enables instant, robust, sutureless GP sealing. In vivo rodent and porcine models demonstrated PPCL@Mg hydrogel's efficacy in sealing gastric perforations and accelerating healing. This dry powder system presents a promising strategy for GP repair with significant translational potential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


