生物工程支架在脊髓损伤中的神经再生和神经保护作用:临床前和早期临床研究的系统回顾。
IF 2.2
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
摘要
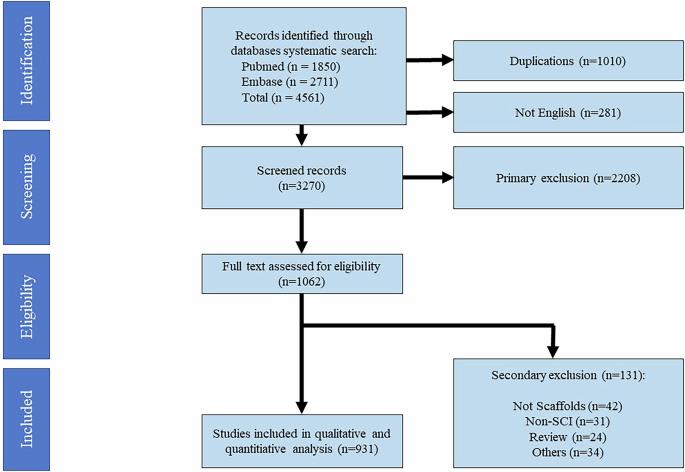
研究设计:系统评价目的:系统地检查在脊髓损伤(SCI)的临床前动物模型和人类研究中使用生物工程支架,有无生物活性剂、药物或细胞移植。方法:遵循PRISMA指南进行系统评价和荟萃分析,并在PROSPERO注册(ID: CRD42023437266)。2023年8月27日,MEDLINE和Embase进行了全面检索,确定了支架作为神经再生和神经保护治疗SCI的研究。人类研究使用ROBINS-I进行评估,荟萃分析侧重于临床结果。结果:在筛选的4561篇文章中,包括931项研究:体内(82%)、体外(16%)和人体研究(1%)。分析了各种生物材料(N = 82,天然:38%,合成:62%)、细胞类型(N = 27, NSCs: 24%,雪旺细胞:13%,npc: 12%)、生物活性药物(N = 38, NT-3: 32%, BDNF: 25%, FGF: 24%)和药理药物(N = 88, chABC: 12%,肝素:11%,紫杉醇:10%)。14项人体研究包括急性和慢性SCI患者,其中颈椎(36%)和胸椎(64%)。临床试验显示为中低质量(ROBINS-I)。我们的荟萃分析显示,合并的2量表AIS转换率为30.59% (p = 0.005),从急性SCI的33.46% (p = 0.036)到慢性SCI的28.35% (p = 0.084)。此外,合并的1量表AIS转换率为11.79% (p = 0.011),从急性SCI的17.31% (p = 0.131)到慢性SCI的7.44% (p = 0.081)。结论:支架植入具有良好的神经再生潜力,在人体研究中AIS等级的改善证明了这一点。支架正在从实验室研究迅速发展到临床试验,扩大了脊髓损伤的治疗选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Neuroregenerative and neuroprotective effects of bioengineered scaffolds in spinal cord injury: a systematic review of preclinical and early phase clinical studies
Systematic Review To systematically examine the use of bioengineered scaffolds, with/without bioactive agents, drugs, or cellular transplants in preclinical animal models and human studies of spinal cord injury (SCI). Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences A systematic review and meta-analysis was conducted following PRISMA guidelines and registered in PROSPERO (ID: CRD42023437266). A comprehensive search in MEDLINE and Embase on 8/27/2023 identified studies on scaffolds as neuroregenerative and neuroprotective treatments for SCI. Human studies were assessed using ROBINS-I, and meta-analysis focused on clinical outcomes. Of 4561 articles screened, 931 studies were included: in-vivo (82%), in-vitro (16%), and human studies (1%). Various biomaterials (N = 82; natural: 38%; synthetic: 62%), cell types (N = 27; NSCs: 24%, Schwann cells: 13%, NPCs: 12%), bioactive agents (N = 38; NT-3: 32%, BDNF: 25%, FGF: 24%), and pharmacological agents (N = 88; chABC: 12%, heparin: 11%, taxels: 10%) were analyzed. Fourteen human studies included acute and chronic SCI patients, with cervical (36%) and thoracic SCI (64%). Clinical trials demonstrated moderate to low quality (ROBINS-I). Our meta-analysis indicated that the pooled 2-scale AIS conversion rate was 30.59% (p = 0.005) ranging from 33.46% (p = 0.036) in acute to 28.35% (p = 0.084) in chronic SCI. Furthermore, the pooled 1-scale AIS conversion rate was 11.79% (p = 0.011), spanning from 17.31% (p = 0.131) in acute to 7.44% (p = 0.081) in chronic SCI. Scaffold implantation shows promising neuroregenerative potential, evidenced by AIS grade improvement in human studies. Scaffolds are advancing rapidly from laboratory research to clinical trials, expanding treatment options for SCI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Spinal cord
医学-临床神经学
CiteScore
4.50
自引率
9.10%
发文量
142
审稿时长
2 months
期刊介绍:
Spinal Cord is a specialised, international journal that has been publishing spinal cord related manuscripts since 1963. It appears monthly, online and in print, and accepts contributions on spinal cord anatomy, physiology, management of injury and disease, and the quality of life and life circumstances of people with a spinal cord injury. Spinal Cord is multi-disciplinary and publishes contributions across the entire spectrum of research ranging from basic science to applied clinical research. It focuses on high quality original research, systematic reviews and narrative reviews.
Spinal Cord''s sister journal Spinal Cord Series and Cases: Clinical Management in Spinal Cord Disorders publishes high quality case reports, small case series, pilot and retrospective studies perspectives, Pulse survey articles, Point-couterpoint articles, correspondences and book reviews. It specialises in material that addresses all aspects of life for persons with spinal cord injuries or disorders. For more information, please see the aims and scope of Spinal Cord Series and Cases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


