激光闪光熔化低温电镜样品克服择优取向。
IF 32.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
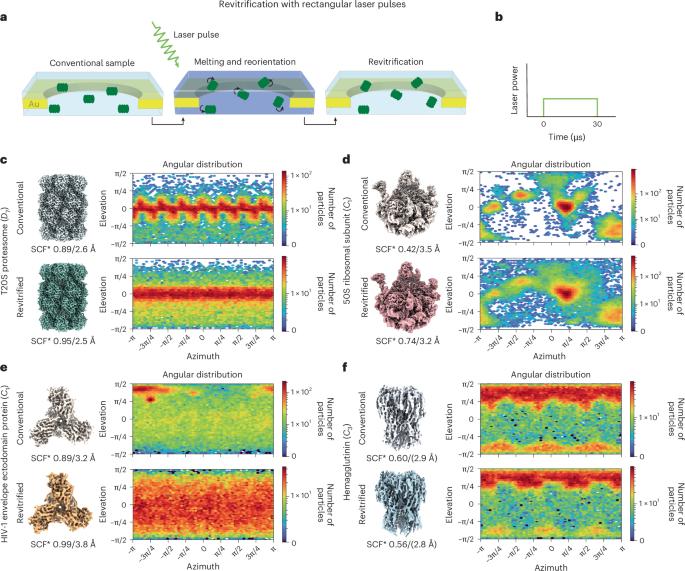
样品制备仍然是低温电子显微镜测定蛋白质结构的瓶颈。一个经常遇到的问题是蛋白质以有限的方向吸附在样品的空气-水界面上。这使得获得高分辨率重建具有挑战性,甚至可能导致项目完全失败。我们以前已经观察到,激光闪光熔化和冷冻电镜样品的再玻璃化降低了大的,对称的颗粒的首选取向。在这里,我们证明了我们的方法实际上可以用来打乱一系列大小和对称的蛋白质的方向。对于某些蛋白质,可以通过在闪融过程中增加加热速率或在再玻璃化之前在样品上沉积无定形冰来增强这种效果。这也使我们能够阐明潜在的机制。我们的实验建立了一组工具,用于克服可以轻松集成到现有工作流中的首选方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Laser flash melting cryo-EM samples to overcome preferred orientation
Sample preparation remains a bottleneck for protein structure determination by cryo-electron microscopy. A frequently encountered issue is that proteins adsorb to the air–water interface of the sample in a limited number of orientations. This makes it challenging to obtain high-resolution reconstructions, or may even cause projects to fail altogether. We have previously observed that laser flash melting and revitrification of cryo-EM samples reduces preferred orientation for large, symmetric particles. Here we demonstrate that our method can in fact be used to scramble the orientation of proteins of a range of sizes and symmetries. The effect can be enhanced for some proteins by increasing the heating rate during flash melting or by depositing amorphous ice onto the sample prior to revitrification. This also allows us to shed light onto the underlying mechanism. Our experiments establish a set of tools for overcoming preferred orientation that can be easily integrated into existing workflows. Individual proteins tend to adopt preferred orientations when subjected to vitrification for cryo-electron microscopy analysis. A laser flash melting procedure followed by rapid revitrification provides a simple approach to mitigate this issue, reducing the number of micrographs required for successful structure determination at high-resolution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Methods
生物-生化研究方法
CiteScore
58.70
自引率
1.70%
发文量
326
审稿时长
1 months
期刊介绍:
Nature Methods is a monthly journal that focuses on publishing innovative methods and substantial enhancements to fundamental life sciences research techniques. Geared towards a diverse, interdisciplinary readership of researchers in academia and industry engaged in laboratory work, the journal offers new tools for research and emphasizes the immediate practical significance of the featured work. It publishes primary research papers and reviews recent technical and methodological advancements, with a particular interest in primary methods papers relevant to the biological and biomedical sciences. This includes methods rooted in chemistry with practical applications for studying biological problems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


