微塑料通过nat10介导的FASN-PI3K/AKT信号的表观遗传失调损害伤口愈合。
IF 5.5
3区 环境科学与生态学
Q2 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
微塑料是具有潜在健康风险的环境污染物。本研究考察了MPs对糖尿病和非糖尿病小鼠伤口愈合的影响。MPs暴露显著延迟伤口愈合,尤其是糖尿病小鼠,伴有表皮厚度减少和胶原沉积受损。在机制上,MPs抑制细胞增殖、血管生成和增加凋亡。转录组学分析确定了关键伤口愈合通路的失调,特别是那些涉及炎症、细胞外基质重塑和脂质代谢的通路。值得注意的是,PI3K/AKT信号通路被抑制。利用人真皮成纤维细胞进行的体外实验证实,MPs破坏了PI3K/AKT通路,减少了细胞的增殖和迁移。进一步的研究表明,MPs抑制n -乙酰转移酶10 (NAT10)的表达,导致Fasn mRNA的ac4c依赖性稳定性降低,从而减少脂质合成,进一步抑制PI3K/AKT通路。我们的研究结果揭示了MPs与糖尿病之间在损伤伤口愈合方面的一种新的相互作用,并提示NAT10-FASN-PI3K/AKT轴可能是一个潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
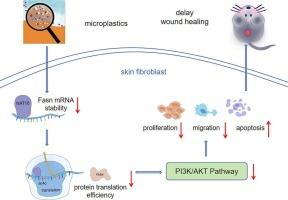
Microplastics impair wound healing via NAT10-mediated epigenetic dysregulation of FASN-PI3K/AKT signaling
Microplastics (MPs) are environmental pollutants with potential health risks. This study examined the effect of MPs on wound healing in both diabetic and non-diabetic mice. MPs exposure significantly delayed wound healing, particularly in diabetic mice, with reduced epidermal thickness and impaired collagen deposition. Mechanistically, MPs suppressed cell proliferation, angiogenesis, and increased apoptosis. Transcriptomic analysis identified dysregulation of critical wound healing pathways, especially those involved in inflammation, extracellular matrix remodeling, and lipid metabolism. Notably, the PI3K/AKT signaling pathway was inhibited. In vitro experiments using human dermal fibroblasts confirmed that MPs disrupted the PI3K/AKT pathway, reducing cell proliferation and migration. Further investigation revealed that MPs suppressed N-acetyltransferase 10 (NAT10) expression, leading to reduced ac4C-dependent stabilization of Fasn mRNA, which in turn diminished lipid synthesis and further inhibited the PI3K/AKT pathway. Our findings reveal a novel interaction between MPs and diabetes in impairing wound healing and suggest the NAT10-FASN-PI3K/AKT axis as a potential therapeutic target.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NanoImpact
Social Sciences-Safety Research
CiteScore
11.00
自引率
6.10%
发文量
69
审稿时长
23 days
期刊介绍:
NanoImpact is a multidisciplinary journal that focuses on nanosafety research and areas related to the impacts of manufactured nanomaterials on human and environmental systems and the behavior of nanomaterials in these systems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


