依普沙坦通过阻断AT1R/SNS/HMGB1和调节PDL-1减轻小鼠外伤性脑损伤多器官功能障碍综合征。
IF 2.4
3区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
外伤性脑损伤不仅局限于大脑,延迟的继发性事件通过交感神经活动、炎症和免疫抑制等三种体内平衡机制的强烈反应扰乱终末器官的功能。目前研究采用减重模型诱导瑞士白化小鼠TBI。在小鼠损伤后30-45 min口服依泊沙坦,剂量分别为0.35 mg/kg和0.7 mg/kg。对小鼠进行神经行为改变测试,并在损伤的急性和慢性阶段切除多个器官,包括脑、心、肺、肝和肾,以进行进一步的水肿、生化、炎症、儿茶酚胺、基因表达和组织病理学评估。结果显示,Epro改善了神经行为表现,维持了血脑屏障和肺完整性。对接研究显示Epro对HMGB1和PDL-1具有较强的结合亲和力,这进一步得到了低组织HMGB1和血清IL-6和TNF-α细胞因子水平的支持,从而阻止了全身性高炎症。此外,Epro治疗通过稳定血清生物标志物,降低血浆去甲肾上腺素水平,使交感神经风暴消退,成功恢复了心脏、肝脏和肾脏功能。急性期各脏器细胞形态异常,而Epro可逆转慢性期脏器细胞形态的变化。此外,epro还促进了调节免疫反应的组织中PD-1/PDL-1和IL-10基因的表达。由此可见,Epro对MODS的器官保护作用是通过抑制AT1/SNS通路发挥的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
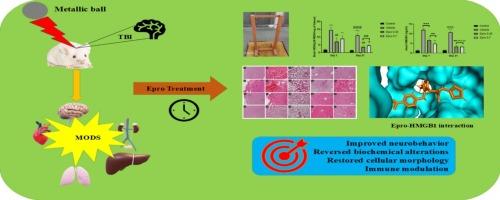
Eprosartan alleviates the traumatic brain injury-induced multi-organ dysfunction syndrome in mice via AT1R/SNS/HMGB1 blockade and PDL-1 modulation
Traumatic brain injury is not constrained only to the brain but delayed secondary events disturb the end organ functioning via intense response of three homeostatic mechanisms such as sympathetic activity, inflammation, and immunosuppression. Current study involved weight drop model to induce TBI in Swiss albino mice. Eprosartan was administered orally after 30–45 min post injury to mice in 0.35 mg/kg and 0.7 mg/kg doses. Mice were tested for neurobehavioral alterations and multiple organs, including brain, heart, lungs, liver, and kidney were excised for further edema, biochemical, inflammatory, catecholamine, gene expression and histopathological estimations at both acute and chronic phases of injury. Results highlighted that Epro improved neurobehavioral performance, maintained the BBB and lung integrity. It also ameliorated the oxidative stress as well as docking studies exhibited strong binding affinity of Epro for HMGB1 and PDL-1, that further supported by low tissue HMGB1 and serum IL-6 and TNF-α cytokines levels which halted the systemic hyperinflammation. Moreover, Epro treatment successfully restored the cardiac, hepatic and kidney function through stabilized serum biomarkers with declined plasma noradrenaline levels that subsides the sympathetic storm. Considerably, a bizarre cellular morphology was displayed by the organs in acute phase of injury whereas Epro reversed the morphological changes at chronic stage. Also, epro encouraged the PD-1/PDL-1 and IL-10 gene expression in the tissues that regulates immune response. Thus, it is concluded that Epro exerts its organ protective effect against MODS via AT1/SNS pathway inhibition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.60
自引率
0.00%
发文量
65
审稿时长
37 days
期刊介绍:
Molecular and Cellular Neuroscience publishes original research of high significance covering all aspects of neurosciences indicated by the broadest interpretation of the journal''s title. In particular, the journal focuses on synaptic maintenance, de- and re-organization, neuron-glia communication, and de-/regenerative neurobiology. In addition, studies using animal models of disease with translational prospects and experimental approaches with backward validation of disease signatures from human patients are welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


