胆汁酸TUDCA抑制胰高血糖素分泌胰α-细胞涉及S1PR2-PI3K途径。
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
2型糖尿病(T2D)的全球患病率持续上升,预测表明未来几十年将达到惊人的记录。虽然胰腺β细胞功能障碍和胰岛素抵抗是T2D发病的关键因素,但α细胞的损伤也与T2D发病有关。高胰高血糖素血症和胰高血糖素释放抑制改变在T2D患者中经常发现,有助于高血糖。胆汁酸作为一种调节新陈代谢的新型信号分子已经出现。大量证据表明,胆汁酸TUDCA口服治疗T2D具有治疗益处,主要是通过改善β细胞的胰岛素释放和外周组织的胰岛素敏感性。然而,目前尚不清楚TUDCA是否会影响其他参与葡萄糖代谢控制的过程。本研究表明,急性给药TUDCA对小鼠胰岛和释放胰高血糖素的αTC1-9细胞具有胰高血糖素抑制作用。药理和/或分子抑制鞘氨醇-1-磷酸受体2 (S1PR2)和PI3K通路减弱了TUDCA对胰高血糖素释放的作用。此外,TUDCA增加了α-细胞中atp敏感的K+ (KATP)通道的活性,降低了动作电流,抑制了Ca2+信号传导,而不直接影响胞外过程。在高血糖状态下,葡萄糖诱导的胰高血糖素分泌抑制被发现受到损害,而TUDCA能够抑制α-细胞功能,突出其在病理背景下的胰高血糖素抑制作用。总之,这些发现表明,TUDCA诱导的胰高血糖素分泌抑制涉及α-细胞KATP通道的打开和S1PR2/PI3K通路的激活,扩大了TUDCA在糖尿病治疗中的潜在治疗益处。本文章由计算机程序翻译,如有差异,请以英文原文为准。
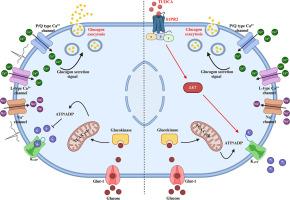
Inhibition of glucagon secretion from pancreatic α-cells by the bile acid TUDCA involves a S1PR2-PI3K pathway
The global prevalence of type 2 diabetes (T2D) continues to rise, and predictions indicate alarming records in coming decades. Although pancreatic β-cell dysfunction and insulin resistance are key factors in the etiology of T2D, the impairment of α-cells has been also implicated. Hyperglucagonemia and altered suppression of glucagon release can be frequently found in individuals with T2D, contributing to hyperglycemia. Bile acids have emerged as novel signaling molecules that regulate metabolism. A wealth of evidence shows that oral treatment with the bile acid TUDCA has therapeutic benefits in T2D, primarily by improving both insulin release from β-cells and insulin sensitivity in peripheral tissues. However, it is unknown whether TUDCA could affect other processes involved in the control of glucose metabolism. Here, we show that acute administration of TUDCA exerts a glucagonstatic action on mouse pancreatic islets and glucagon-releasing αTC1-9 cells. Pharmacological and/or molecular inhibition of the sphingosine-1-phosphate receptor 2 (S1PR2) and the PI3K pathway blunted the TUDCA effect on glucagon release. Additionally, TUDCA increased the activity of ATP-sensitive K+ (KATP) channels, decreased action currents and inhibited Ca2+ signaling in α-cells without directly affecting the exocytotic process. Glucose-induced suppression of glucagon secretion was found to be compromised under hyperglycemic conditions, yet TUDCA was able to inhibit α-cell function, highlighting its glucagonstatic effect in a pathological context. Collectively, these findings suggest that TUDCA-induced inhibition of glucagon secretion involves the opening of α-cell KATP channels and activation of the S1PR2/PI3K pathway, expanding the repertoire of potential therapeutic benefits of TUDCA in diabetes treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


