Taxifolin对抑制PI3K/AKT和MAPK信号的细菌性脑膜炎的治疗作用
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-08-23
DOI:10.1016/j.ijantimicag.2025.107601
引用次数: 0
摘要
背景:细菌性脑膜炎(BM)是一种以高死亡率和神经系统后遗症为特征的严重传染病。抗菌素耐药性的日益普遍影响了抗生素治疗,强调迫切需要替代治疗。Taxifolin (TAX)是一种天然存在的类黄酮,具有多种生物活性,但其在BM中的作用尚不清楚。目的:研究TAX对脑脊髓炎的治疗作用,探讨其潜在的分子机制。研究设计和方法:采用体外和体内两种方法,建立了肠外致病性大肠杆菌和猪链球菌的BM模型。通过综合分析病理变化和炎症反应来评价TAX的治疗效果。通过网络药理学、Western blotting、药物亲和靶稳定性实验、细胞热移实验等多种方法研究其分子机制。结果:TAX在体外显著抑制细菌诱导的小胶质细胞和脑内皮细胞的细胞死亡,减少小胶质细胞的炎症反应,并保护内皮紧密连接蛋白。在小鼠中,TAX显著降低了死亡率,减轻了组织损伤,抑制了炎症反应。TAX的保护作用与抑制PI3K/AKT和MAPK通路有关,AKT被确定为其直接靶点。结论:我们的研究结果表明,TAX是一种潜在的BM治疗药物,其保护作用与抑制PI3K/AKT和MAPK通路有关。这些结果为进一步发展基于税收的BM干预措施提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
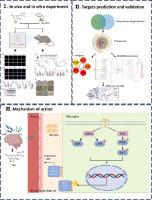
Therapeutic effect of taxifolin on bacterial meningitis associated with inhibition of PI3K/AKT and MAPK signalling
Background
Bacterial meningitis (BM) represents a severe infectious disease characterized by high mortality and neurological sequelae. The increasing prevalence of antimicrobial resistance has compromised antibiotic therapies, emphasizing an urgent need for alternative treatments. Taxifolin (TAX), a naturally occurring flavonoid, exhibits multiple bioactivities, yet its role in BM remains unknown.
Purpose
This study investigated the therapeutic effects of TAX in BM and explored its underlying molecular mechanisms.
Study design and methods
Using both in vitro and in vivo approaches, we established BM models with extraintestinal pathogenic Escherichia coli and Streptococcus suis. The therapeutic efficacy of TAX was assessed through comprehensive analyses of pathological changes and inflammatory responses. Molecular mechanisms were investigated using multiple approaches, including network pharmacology, Western blotting, drug affinity-responsive target stability assay, and cellular thermal shift assay.
Results
TAX significantly inhibited bacteria-induced cell death in microglia and brain endothelial cells, reduced microglial inflammatory responses, and protected endothelial tight junction proteins in vitro. In mice, TAX markedly reduced mortality, alleviated tissue damage, and suppressed inflammatory responses. The protective effects of TAX were associated with the inhibition of PI3K/AKT and MAPK pathways, with AKT identified as its direct target.
Conclusions
Our findings identify TAX as a potential therapeutic agent for BM treatment, with its protective effects associated with the inhibition of PI3K/AKT and MAPK pathways. These results provide a foundation for the further development of TAX-based interventions against BM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


