转录组学分析揭示了四溴双酚a诱导的鲤鱼肝脏脂质稳态失调。
IF 4.3
3区 环境科学与生态学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology C-toxicology & Pharmacology
Pub Date : 2025-08-26
DOI:10.1016/j.cbpc.2025.110336
引用次数: 0
摘要
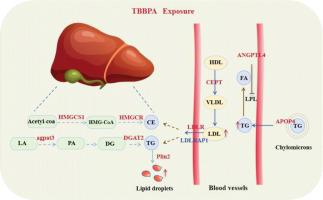
TBBPA是一种普遍存在的溴化阻燃剂,具有生物蓄积性和毒性,对生态环境具有重大危害。以前的研究主要集中在非靶向代谢组学和脂质组学上,以表征TBBPA对代谢终产物的影响。然而,关于tbbpa诱导的水生生物脂质代谢破坏的机制的数据有限。本研究通过综合生化、组织化学评估和转录组学分析,系统地研究了TBBPA对鲤鱼脂质代谢的剂量依赖性作用。鱼暴露于与环境相关的TBBPA浓度(0、0.005、0.05和0.5 mg/L)中14天。血清生化分析显示剂量依赖性高脂血症,表现为TG和T-CHO水平升高,SOD和CAT活性抑制,MDA水平升高。尽管肝内TG/T-CHO含量无显著变化(P > 0.05),但油红O染色显示肝脂滴积聚,其中0.5 mg/L组表现出最严重的组织病理表型。转录组学分析发现AMPK和PPAR信号通路是tbbpa诱导的代谢失调的关键调节因子。低剂量TBBPA暴露(0.05 mg/L)下,脂质积累触发AMPK/PPAR-α/CPT1轴代偿激活,增强脂肪酸β-氧化,部分抵消脂质沉积。相反,高剂量TBBPA(0.5 mg/L)通过协调上调与脂质合成、运输和储存相关的基因来破坏脂质代谢,最终导致病理性脂质沉积。这项研究为水生物种中tbbpa诱导的代谢失调的分子级联提供了重要的见解,强调了脂质稳态的剂量依赖性破坏。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Transcriptomic profiling reveals tetrabromobisphenol A-induced dysregulation of hepatic lipid homeostasis in common carp (Cyprinus carpio)
TBBPA, a ubiquitously distributed brominated flame retardant, poses significant ecological risks due to its bioaccumulative potential and toxicity. Previous investigations have predominantly focused on untargeted metabolomics and lipidomics to characterize the effects of TBBPA on metabolic end products. However, limited data are available regarding the mechanisms underlying TBBPA-induced disruption of lipid metabolism in aquatic organisms. This study systematically investigated the dose-dependent effects of TBBPA on lipid metabolism in common carp through integrated biochemical, histochemical evaluations, and transcriptomic profiling. Fish were exposed to environmentally relevant TBBPA concentrations (0, 0.005, 0.05, and 0.5 mg/L) for a 14-day duration. Serum biochemical analyses revealed dose-dependent hyperlipidemia, manifested by elevated TG and T-CHO levels, suppressed SOD and CAT activities, and increased MDA levels. Oil Red O staining demonstrated hepatic lipid droplet accumulation despite no significant (P > 0.05) alterations in intrahepatic TG/T-CHO content, with the 0.5 mg/L group exhibited the most severe histopathological phenotype. Transcriptomic profiling identified the AMPK and PPAR signaling pathways as pivotal regulators of TBBPA-induced metabolic dysregulation. Under low-dose TBBPA exposure (0.05 mg/L), lipid accumulation triggered compensatory activation of the AMPK/PPAR-α/CPT1 axis, enhancing fatty acid β-oxidation to partially counteract lipid deposition. Conversely, high-dose TBBPA (0.5 mg/L) disrupted lipid metabolism through coordinated upregulation of genes associated with lipid synthesis, transport, and storage, culminating in pathological lipid deposition. This study provides critical insights into the molecular cascades underlying TBBPA-induced metabolic dysregulation in aquatic species, highlights the dose-dependent disruption of lipid homeostasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.50
自引率
5.10%
发文量
206
审稿时长
30 days
期刊介绍:
Part C: Toxicology and Pharmacology. This journal is concerned with chemical and drug action at different levels of organization, biotransformation of xenobiotics, mechanisms of toxicity, including reactive oxygen species and carcinogenesis, endocrine disruptors, natural products chemistry, and signal transduction with a molecular approach to these fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


