CBX2和EZH2通过H3k27的三甲基化抑制铁下沉,共同参与胃癌5-Fu耐药。
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
背景与目的:染色体盒2 (CBX2)在多种癌症的恶性表型中起着关键作用,在胃癌(GC)中被发现表达上调。本研究旨在探讨CBX2在GC中的具体功能及其机制。方法:基于在线生物信息学分析,揭示CBX2在GC中的潜在作用。免疫组织化学、qRT-PCR、western blot检测蛋白和mRNA表达水平。采用细胞转染技术、CCK-8试剂盒、集落形成实验、流式细胞术、商品化试剂盒及co-IP等方法,探讨CBX2对5-Fu耐药的影响及机制。采用异种移植瘤裸鼠模型进行体内实验。结果:CBX2在胃癌患者肿瘤组织中表达上调,与化疗耐药呈正相关,与EZH2表达呈正相关。下调CBX2可使5-Fu抗性GC细胞对5-Fu重敏,而过表达CBX2可通过调控铁凋亡增强GC细胞对5-Fu的抗性。体内实验表明,CBX2过表达增强了肿瘤中5-Fu的敏感性。机制上,CBX2和EZH2通过诱导H3k27 (H3k27me3)的三甲基化共同抑制GC细胞的铁凋亡。抑制EZH2可阻断CBX2过表达对GC细胞5-FU敏感性的诱导作用,降低CBX2过表达GC细胞的铁凋亡和H3k27me3水平。结论:CBX2/EZH2通过H3k27me3抑制铁凋亡,协同赋予GC细胞5-FU耐药性,可能是一种有前景的GC治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
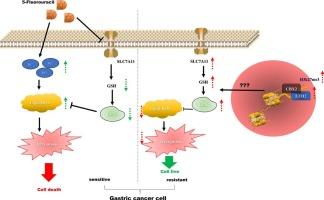
CBX2 and EZH2 cooperatively contribute to 5-Fu resistance in gastric cancer by suppressing ferroptosis via trimethylation of H3k27
Background and objective
Chromobox 2 (CBX2) plays a pivotal role in the malignant phenotypes of several cancers, which has been found to up-regulated in gastric cancer (GC). This study aimed to investigate the specific function and underlying mechanism of CBX2 in GC.
Methods
The potential role of CBX2 in GC was uncovered based on online bioinformatic analysis. The protein and mRNA expression levels were assessed by immunohistochemistry, qRT-PCR, and western blot. Using parental and the corresponding resistant GC cell lines, the effect and mechanism of CBX2 on 5-Fu resistance were explored by cell transfection technique, CCK-8 kit, colony formation assay, flow cytometry, the commercial kit, as well as co-IP. Xenograft tumor nude mice models were applied for in vivo assay.
Results
The expression of CBX2 was up-regulated in tumor tissues of GC patients and positively correlated with chemo-resistance, as well as that of EZH2. Knockdown of CBX2 resensitized 5-Fu resistant GC cells to 5-Fu while overexpressing CBX2 enhance GC cells against 5-Fu via the regulation of ferroptosis. In vivo experiments demonstrated that CBX2 overexpression enhanced 5-Fu sensitivity in tumors. Mechanically, CBX2 and EZH2 cooperatively suppressed ferroptosis in GC cells by inducing trimethylation of H3k27 (H3k27me3). Suppressing EZH2 blocked the inductive effect of CBX2 overexpression on 5-FU sensitivity of GC cells and reduced ferroptosis and H3k27me3 levels in CBX2 overexpressed GC cells.
Conclusion
CBX2/EZH2 cooperatively confers 5-FU resistance in GC cells through suppressing ferroptosis via H3k27me3, which may lead to a promising therapeutic strategy for GC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


