通过电解质工程定制界面质子耦合电子转移,用于高选择性H2O2生产。
IF 21.1
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
通过电解液设计对双电层进行定向调制已成为控制电化学反应途径的一种变革性策略。虽然氧还原反应(ORR)代表了电解质效应明显的典型例子,但电解质介导的界面微环境调节的原子尺度机制仍然不完全清楚。在这里,我们阐明了乙腈(ACN)添加剂如何调整碱性ORR途径,在碳催化剂上选择性地电合成H2O2。通过综合分子动力学模拟、原位光谱和电化学阻抗分析,我们证明ACN优化了三相界面以增强ORR活性,并通过取代阳离子溶剂壳中的水和破坏水分子的h键连续性来重构界面水环境。这些协同效应有效地减轻了界面质子/电子泛洪,同时优化了质子耦合电子转移动力学,与未经修饰的KOH体系(60%)相比,H2O2选择性显著提高(90%)。通过建立电解质组成与界面微环境和反应途径的构效关系,为可持续电合成H2O2提供了一种新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
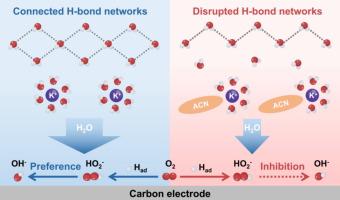
Tailoring interfacial proton-coupled electron transfer via electrolyte engineering for high-selectivity H2O2 production
The targeted modulation of electric double layer through electrolyte design has emerged as a transformative strategy for controlling electrochemical reaction pathways. While the oxygen reduction reaction (ORR) represents a paradigmatic example where electrolyte effects are pronounced, the atomic-scale mechanisms underlying electrolyte-mediated regulation of interfacial microenvironments remain incompletely understood. Here, we elucidate how acetonitrile (ACN) additive tailors the alkaline ORR pathway toward selective H2O2 electrosynthesis on carbon catalysts. Through integrated molecular dynamics simulations, in situ spectroscopy, and electrochemical impedance analysis, we demonstrate that ACN optimizes the three-phase interface to enhance ORR activity, and restructures interfacial water environments by displacing water in cationic solvation-shell and disrupting H-bonding continuity of water molecules. These synergistic effects effectively mitigate interfacial proton/electron flooding while optimizing proton-coupled electron transfer kinetics, resulting in dramatically enhanced H2O2 selectivity (90%) compared to the unmodified KOH system (60%). By establishing the structure-activity relationship of electrolyte composition with interfacial microenvironment and reaction pathway, this work provides a novel strategy for sustainable H2O2 electrosynthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Bulletin
MULTIDISCIPLINARY SCIENCES-
CiteScore
24.60
自引率
2.10%
发文量
8092
期刊介绍:
Science Bulletin (Sci. Bull., formerly known as Chinese Science Bulletin) is a multidisciplinary academic journal supervised by the Chinese Academy of Sciences (CAS) and co-sponsored by the CAS and the National Natural Science Foundation of China (NSFC). Sci. Bull. is a semi-monthly international journal publishing high-caliber peer-reviewed research on a broad range of natural sciences and high-tech fields on the basis of its originality, scientific significance and whether it is of general interest. In addition, we are committed to serving the scientific community with immediate, authoritative news and valuable insights into upcoming trends around the globe.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


