取代1,2,3-噻二唑作为新型农业硝化抑制剂的构效关系研究。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在农业土壤中使用硝化抑制剂,以保持作物长时间获得铵(NH4+),同时减少硝酸盐(NO3-)的淋失和强效温室气体一氧化二氮(N2O)的排放。不幸的是,由于不太清楚的原因,目前的商业抑制剂在不同的农业生态系统中表现出不一致的性能,这强调了开发新的硝化抑制剂化合物以提高农业环境可持续性的必要性。在这项工作中,我们进行了构效关系(SAR)研究,通过实验室土壤培养探索了12种单取代和双取代1,2,3-噻二唑作为硝化抑制剂的潜力。以一个或两个甲基取代的1,2,3-噻二唑以及具有融合环戊基环的1,2,3-噻二唑表现出最有希望的抑制活性,其抑制效果优于工业硝化抑制剂3,4-二甲基吡唑(DMP)。较大的烷基取代基和极性官能团取代基的抑制活性较差或无抑制活性。这些数据与我们之前对取代的1,2,3-三唑的发现一致,即杂芳烃框架上的短非极性烷基取代基有利于硝化抑制性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
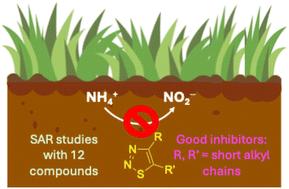
Structure–activity relationship study of substituted 1,2,3-thiadiazoles as novel nitrification inhibitors for agriculture
Nitrification inhibitors are used in agricultural soils to maintain ammonium (NH4+) available for crops for longer periods while reducing leaching of nitrate (NO3−) and emission of the potent greenhouse gas nitrous oxide (N2O). Unfortunately, and for reasons not well understood, the current commercial inhibitors have shown inconsistencies in their performance across various agroecosystems, underscoring the need for the development of new nitrification inhibitor compounds to increase agriculture's environmental sustainability. In this work, we have performed structure–activity relationship (SAR) studies to explore the potential of 12 mono- and disubstituted 1,2,3-thiadiazoles as nitrification inhibitors through laboratory soil incubations. 1,2,3-Thiadiazoles substituted with one or two methyl groups as well as those with a fused cyclopentyl ring showed the most promising inhibitory activities, which can outperform the commercial nitrification inhibitor 3,4-dimethylpyrazole (DMP). Larger alkyl substituents as well as substituents with polar functional groups showed poorer or no inhibitory activity. These data align with our previous findings for substituted 1,2,3-triazoles that short, non-polar alkyl substituents on the heteroaromatic framework are beneficial for nitrification inhibitory properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


