钌催化合成三环1,5-融合1,2,3-三唑哌嗪。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
双环化策略,包括顺序钌催化叠氮化物炔环加成(RuAAC)和借氢,允许在脯氨酸支架上快速组装三环1,5-融合1,2,3-三唑哌嗪。在温和的反应条件下,初始的ruaac环化反应的产率可达99%,而循环的借氢反应可获得所需的融合三唑哌嗪,产率可达75%。这两个反应步骤都是高度原子经济的,我们设想三环产物可以在不对称转化中找到应用,以及在生物利益产物的合成中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
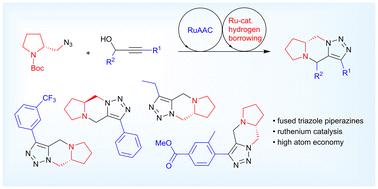
Ruthenium-catalyzed synthesis of tricyclic 1,5-fused 1,2,3-triazole piperazines
A double cyclization strategy, involving sequential ruthenium-catalyzed azide alkyne cycloaddition (RuAAC) and hydrogen borrowing, allows the rapid assembly of tricyclic 1,5-fused 1,2,3-triazole piperazines from a proline scaffold. The initial RuAAC-cyclization proceeded in up to 99% yield under mild reaction conditions, while cyclative hydrogen borrowing afforded the desired fused triazole piperazines in up to 75% yield. Both reaction steps are highly atom economic and we envisage that the tricyclic products can find applications in asymmetric transformations, as well as in the synthesis of products of biological interest.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


