可见光诱导邻芳基苯甲醛分子内脱氢环化。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用稳定的n -氨基吡啶盐作为HAT试剂和温和氧化剂,制备了邻芳基苯甲醛在可见光下的脱氢环化反应,得到了多种取代模式的芴酮,收率中等至优异。此外,该方案适用于天然产物诺比隆的从头合成。机理研究支持光诱导n -氨基吡啶盐的同裂和自由基链过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
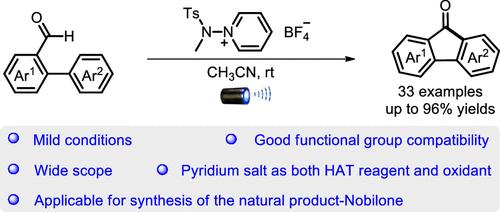
Visible-Light-Induced Intramolecular Dehydrogenative Cyclization of o-Aryl Benzaldehydes
A visible-light-induced dehydrogenative cyclization of o-aryl benzaldehydes has been developed by using bench-stable N-aminopyridinium salt as both a HAT reagent and a mild oxidant, which affords a wide range of fluorenones with diverse substitution patterns in moderate to excellent yields. Furthermore, this protocol is applicable to the de novo synthesis of the natural product Nobilone. Mechanism investigation supports a photoinduced homocleavage of N-aminopyridinium salt and a radical chain process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


