移植肾小球炎的免疫微环境
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
移植肾小球炎是一种在同种异体肾移植排斥反应中可见的形态学病变,与不良预后相关;然而,关于免疫细胞如何在肾小球内浸润和特异组织知之甚少。方法采用CODEX多荧光成像技术对41例同种异体肾切除术患者的52个蛋白标志物进行回顾性检测,评价移植肾小球炎的免疫景观。结果对炎性肾小球内18种不同类型的免疫细胞进行了表征,与肾小球外微环境相比,这些免疫细胞具有独特的表型和组成。免疫表型在个体肾小球中是保守的,与一般损伤状态有关,M1巨噬细胞和效应CD8 T细胞与轻度炎症有关。基于距离的空间分析进一步揭示了内皮细胞中心周围由M2巨噬细胞、记忆性CD8 T细胞和耗竭性CD8 T细胞组成的纤维化群落。这些相互作用网络与不良肾小球重构区域、纤维化蛋白的表达有关,并且在c4d阳性排斥反应患者中更为普遍。结论:这些结果表明移植肾小球炎期间不同的细胞间相互作用是同种免疫损伤和慢性重构的标志,并可能为临床排斥综合征的组织学风险评估提供新的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
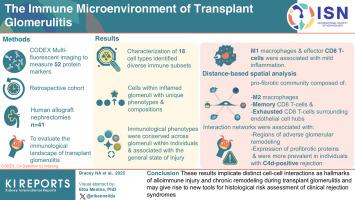
The Immune Microenvironment of Transplant Glomerulitis
Introduction
Transplant glomerulitis is a morphological lesion seen in kidney allograft rejection that is associated with poor outcomes; however, little is known about how immune cells infiltrate and organize specifically within glomeruli.
Methods
We used Co-Detection by Indexing (CODEX) multifluorescent imaging to measure 52 protein markers in a retrospective cohort of 41 human allograft nephrectomies (ANs) and evaluated the immunological landscape of transplant glomerulitis.
Results
Characterization of 18 cell types identified diverse immune cells within inflamed glomeruli, with unique phenotypes and compositions compared with the extraglomerular microenvironment. Immunological phenotypes were conserved across glomeruli within individuals and associated with the general state of injury, with M1 macrophages and effector CD8 T cells associated with mild inflammation. Distance-based spatial analysis further revealed a profibrotic community composed of M2 macrophages, memory CD8 T cells and exhausted CD8 T cells surrounding endothelial cell hubs. These interaction networks were associated with regions of adverse glomerular remodeling, expression of profibrotic proteins, and were more prevalent in individuals with C4d-positive rejection.
Conclusion
These results implicate distinct cell-cell interactions as hallmarks of alloimmune injury and chronic remodeling during transplant glomerulitis and may give rise to new tools for histological risk assessment of clinical rejection syndromes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


