单核rna测序鉴定原发性与适应性不良局灶节段性肾小球硬化中壁上皮细胞的差异纤维化反应
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
局灶节段性肾小球硬化(FSGS)病变发生在多种临床情况下,均以严重足细胞损伤为特征。由于缺乏对其病理生理差异的了解,缺乏可靠的生物标志物,因此区分原发性和非适应性形式的FSGS仍然具有挑战性。方法采用单核rna测序(snRNA-seq)技术鉴定表型良好的原发性与不适应性FSGS肾脏活检中差异表达的转录特征。我们纳入了来自新诊断的原发性FSGS (n = 9,均为肾病)、不适应性FSGS (n = 9,均为非肾病)、蛋白尿对照组(抗磷脂酶A2受体抗体阳性膜性肾病[PLA2R+ MN], n = 3)和健康对照组(n = 4)的冷冻保存肾活检核心。结果共鉴定出120,751个高质量细胞核,其中足细胞2471个,壁上皮细胞1574个。在原发性FSGS中,足细胞表现出更明显但非特异性的损伤模式,其免疫途径上调,如抗原呈递和哺乳动物雷帕霉素靶蛋白(mTOR)复合物1 (mTORC1)信号传导。肾小球细胞-细胞相互作用分析显示,原发性FSGS PECs中促纤维化TGF-β和PDGFR-β信号传导增加,这也上调了构成正常pecc来源的细胞外基质(ECM)的基因(LAMB1, COL4A1, COL4A5, COL4A6)。在不适应FSGS中,足细胞中差异表达基因(DEGs)较少,以PEC-PEC相互作用为主。在这里,(肌)成纤维细胞样PEC亚群上调非IV型纤维和网络形成胶原(COL1A1, COL5A1, COL8A1),这可能进一步促进肾小球硬化的发展。本研究展示了表型良好的FSGS患者的单细胞转录图谱,并为原发性与非适应性FSGS的纤维化性PEC反应差异提供了证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
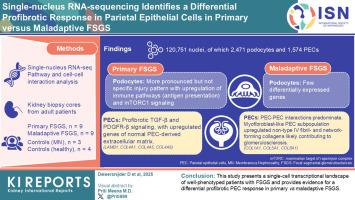
Single-Nucleus RNA-Sequencing Identifies a Differential Profibrotic Response in Parietal Epithelial Cells in Primary Versus Maladaptive Focal Segmental Glomerulosclerosis
Introduction
Focal segmental glomerulosclerosis (FSGS) lesions occur in a wide range of clinical conditions that are all characterized by critical podocyte injury. Differentiating primary from maladaptive forms of FSGS remains challenging because of the absence of reliable biomarkers, resulting from a lack of insight into their pathophysiological differences.
Methods
We used single-nucleus RNA-sequencing (snRNA-seq) to identify differentially expressed transcriptional signatures in kidney biopsies of well-phenotyped primary versus maladaptive FSGS. We included cryopreserved kidney biopsy cores from adult patients with newly diagnosed primary FSGS (n = 9, all nephrotic), maladaptive FSGS (n = 9, all nonnephrotic), proteinuric controls (antiphospholipase A2 receptor antibody–positive membranous nephropathy [PLA2R+ MN], n = 3), and healthy controls (n = 4).
Results
We identified 120,751 high-quality nuclei, including 2471 podocytes and 1574 parietal epithelial cells (PECs). In primary FSGS, podocytes showed a more pronounced but not specific injury pattern with upregulation of immune pathways, such as antigen presentation, and mammalian target of rapamycin (mTOR) complex 1 (mTORC1)-signaling. Glomerular cell-cell interaction analysis showed increased profibrotic TGF-β and PDGFR-β signaling in primary FSGS PECs, which also upregulated genes that compose the normal PEC-derived extracellular matrix (ECM) (LAMB1, COL4A1, COL4A5, COL4A6). In maladaptive FSGS, podocytes showed few differentially expressed genes (DEGs) and PEC-PEC interactions predominated. Here, a (myo-)fibroblast-like PEC subpopulation upregulated non–type IV fibril- and network-forming collagens (COL1A1, COL5A1, COL8A1), which may further contribute to the development of glomerulosclerosis.
Conclusion
This study presents a single-cell transcriptional landscape of well-phenotyped patients with FSGS and provides evidence for a differential profibrotic PEC response in primary versus maladaptive FSGS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


