足细胞厌氧糖酵解失调与局灶节段性肾小球硬化的进展有关
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
微小改变病(MCD)和局灶节段性肾小球硬化(FSGS)是具有不同临床病程和治疗反应的足细胞病变。FSGS常导致终末期肾脏疾病。因此,它们的异质性需要病例分层和病理生理学的阐明。能量代谢在FSGS发病机制和分层中的作用尚未明确。因此,本研究旨在验证评估能量动力学是否可以作为MCD或FSGS分层的新方法,并探讨能量代谢在MCD或FSGS中的作用。方法用活检证实的MCD或FSGS患者血清处理培养的人足细胞。使用流式细胞术分析血清处理的足细胞凋亡,通过质谱分析代谢组学,并使用细胞外通量分析仪分析实时三磷酸腺苷(ATP)生成率。在足细胞特异性乳酸脱氢酶(LDH) A (LDHA)缺乏的小鼠和对照小鼠中诱导阿霉素引起的肾病。结果与MCD患者相比,FSGS患者血清显著诱导人足细胞凋亡。细胞凋亡严重程度与节段性闭塞和皮质类固醇抵抗有关。代谢组学分析显示,MCD和FSGS患者血清中足细胞的厌氧糖酵解和三羧酸循环(TCA)相关代谢物存在差异。在用FSGS患者血清处理足细胞时,高凋亡率的足细胞糖酵解ATP的产生显著减少。FSGS患者血清抑制LDHA活性,抑制α-肌动蛋白4 (ACTN4)表达,促进足细胞肌动蛋白重塑。与对照小鼠相比,足细胞特异性ldha缺陷小鼠阿霉素肾病的节段性硬化症更为突出。结论fsgs进展与足细胞厌氧糖酵解减少有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
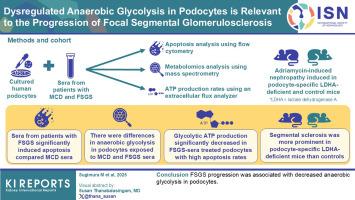
Dysregulated Anaerobic Glycolysis in Podocytes is Relevant to the Progression of Focal Segmental Glomerulosclerosis
Introduction
Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are podocytopathies with varying clinical courses and therapeutic responses. FSGS often leads to end-stage renal disease. Consequently, their heterogeneity requires case stratification and pathophysiological elucidation. The involvement of energy metabolism in FSGS pathogenesis and stratification has not been clarified. Therefore, this study aimed to verify whether evaluating energy kinetics can be a new approach to MCD or FSGS stratification and explore the role of energy metabolism in MCD or FSGS.
Methods
Cultured human podocytes were treated with sera from patients with biopsy-confirmed MCD or FSGS. Serum-treated podocytes were analyzed for apoptosis using flow cytometry, metabolomics via mass spectrometry, and real-time adenosine triphosphate (ATP) production rates using an extracellular flux analyzer. Adriamycin-induced nephropathy was induced in podocyte-specific lactate dehydrogenase (LDH) A (LDHA)-deficient and control mice.
Results
The sera from patients with FSGS significantly induced apoptosis in human podocytes compared with those from individuals with MCD. Apoptosis severity was associated with segmental obliteration and corticosteroid resistance. Metabolomic analysis revealed differences in anaerobic glycolysis and tricarboxylic acid cycle (TCA)-related metabolites in podocytes exposed to the sera of patients with MCD and FSGS. In the podocytes treated with sera from patients with FSGS, glycolytic ATP production significantly decreased in cases with high apoptosis rates. The sera from patients with FSGS suppressed LDHA activity, suppressed α-actinin 4 (ACTN4) expression, and promoted actin remodeling of podocytes. Segmental sclerosis was more prominent in podocyte-specific LDHA-deficient mice with adriamycin-induced nephropathy than in control mice.
Conclusion
FSGS progression was associated with decreased anaerobic glycolysis in podocytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


