利用家蚕小孢子虫跨膜蛋白NbTMP1培育抗psm抗性家蚕
IF 2.4
3区 生物学
Q1 ZOOLOGY
引用次数: 0
摘要
由家蚕微孢子虫引起的psambline病是对养蚕业的重大挑战。为了提高家蚕的抗性,我们培育了一株表达单链片段可变抗体F12 (scFvF12)的转基因菌株(N-F12),靶向家蚕关键的跨膜蛋白NbTMP1。该抗体与Slmb蛋白n端F-box结构域泛素化标记融合,通过宿主的泛素-蛋白酶体系统(UPS)促进NbTMP1的降解。Western blot分析证实重组NSlmb::scFvF12抗体能够特异性识别和标记NbTMP1,导致其降解。此外,在N-F12转基因细胞中,家蚕的增殖明显受到抑制。表达N-F12的转基因家蚕对家蚕表现出明显的抗性,在不影响关键经济性状的情况下获得更高的成活率。这项研究展示了一种利用寄主的UPS降解病原体蛋白质的新策略,在蚕桑养殖和更广泛的寄主-病原体系统中具有潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
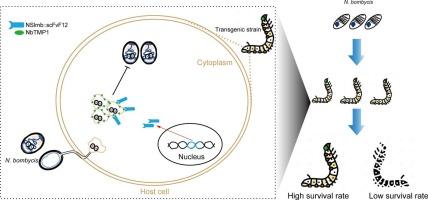
Developing Pébrine-Resistant silkworms through targeting the transmembrane protein NbTMP1 in Nosema bombycis
Pébrine disease, caused by the microsporidium Nosema bombycis, represents a significant challenge to the sericulture industry. To enhance the resistance of silkworm, we developed a transgenic strain (designated N-F12) expressing a single-chain fragment variable antibody F12 (scFvF12), targeting the critical transmembrane protein NbTMP1 of N. bombycis. The antibody was fused with the ubiquitination tag, the F-box domain at the N-terminal of Slmb protein (NSlmb), facilitating the degradation of NbTMP1 via the host’s ubiquitin–proteasome system (UPS). Western blot analysis confirmed that the recombinant NSlmb::scFvF12 antibody can specifically recognize and label NbTMP1, leading to its degradation. Additionally, the proliferation of N. bombycis was significantly suppressed in N-F12 transgenic cells. Transgenic silkworms expressing N-F12 exhibited obvious resistance to N. bombycis, achieving higher survival rates without compromising key economic traits. This study demonstrates a novel strategy for pathogen resistance by utilizing the host’s UPS to degrade pathogen proteins, with potential applications in sericulture and broader host-pathogen systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
5.90%
发文量
94
审稿时长
1 months
期刊介绍:
The Journal of Invertebrate Pathology presents original research articles and notes on the induction and pathogenesis of diseases of invertebrates, including the suppression of diseases in beneficial species, and the use of diseases in controlling undesirable species. In addition, the journal publishes the results of physiological, morphological, genetic, immunological and ecological studies as related to the etiologic agents of diseases of invertebrates.
The Journal of Invertebrate Pathology is the adopted journal of the Society for Invertebrate Pathology, and is available to SIP members at a special reduced price.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


