锂离子电池负极材料用新型电化学剥离法合成的石墨烯吸附Sr(Ⅱ)和Cs(Ⅰ)
IF 2.1
3区 环境科学与生态学
Q3 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
由于137Cs和90Sr等放射性核素,向海洋排放核废水可能造成重大的环境和健康风险。因此,有效去除这些核素已成为放射性废水处理领域的一个焦点。传统的恢复方法依赖于物理和化学干预以及生物修复,经济上负担沉重,不适合大规模的恢复工作。废锂离子电池(LIBs)的废石墨也是一个重新利用的挑战,但在双碳目标的背景下,其资源化利用至关重要。在本研究中,利用电化学剥离的方法,利用废锂阳极材料成功合成了层状石墨烯材料。吸附实验表明,石墨烯对Sr(II)和Cs(I)的去除效率随着pH值从1.0增加到6.0而增加,在pH值为6.0时吸附效果更好。吸附动力学表明,Sr(II)和Cs(I)在石墨烯上的吸附符合准二级动力学模型。根据Sips模型计算,在pH 6.0和298 K条件下,对Sr(II)和Cs(I)的最大吸附量分别为73 mg/g和55 mg/g。此外,石墨烯表现出优异的可再生性,在4次循环后仍保持90%以上的吸附容量。吸附机制涉及单层均匀吸附和非均匀吸附的协同作用,其中Sr(II)和Cs(I)占据了石墨烯材料的活性位点。这些研究结果表明,电化学剥落是一种新的有效的石墨烯合成方法,而废lib的废石墨在核废水处理和电化学剥落方面具有很大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
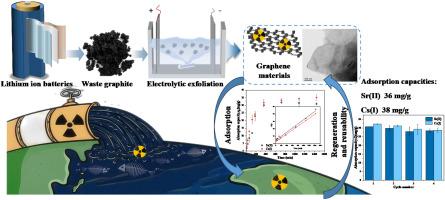
Sr(Ⅱ) and Cs(Ⅰ) adsorbed by graphene synthesized from spent lithium-ion batteries anode materials via novelly electrochemical exfoliation
The discharge of nuclear wastewater into the sea may pose a significant environmental and health risk due to radionuclides such as 137Cs and 90Sr. Consequently, the efficient removal of these nuclides has emerged as a focal point in the field of radioactive wastewater treatment. Traditional restoration methods, which rely on physical and chemical interventions as well as bioremediation, are economically burdensome and unsuitable for large-scale restoration efforts. Waste graphite of spent lithium-ion batteries (LIBs) is also a challenge to repurpose, yet its resourceful utilization is crucial in the context of the dual-carbon goals. In this study, layered graphene materials were successfully synthesized from spent LIBs anode materials by electrochemical exfoliation. Adsorption experiments revealed that the removal efficiency of Sr(II) and Cs(I) by the graphene increases with increasing pH from 1.0 to 6.0, achieving a higher adsorption at pH 6.0. The adsorption kinetics show that the adsorption of Sr(II) and Cs(I) on the graphene fits the pseudo-second-order kinetic model. Calculated from the Sips model, the maximum adsorption capacity for Sr(II) and Cs(I) is determined to be 73 mg/g and 55 mg/g, respectively, at pH 6.0 and 298 K. Furthermore, the graphene exhibited excellent regenerability, maintaining over 90 % of its adsorption capacity after four cycles. The adsorption mechanism involved a synergistic effect of monolayer uniform adsorption and non-uniform adsorption, where Sr(II) and Cs(I) occupied the active sites of the graphene material. These findings suggest that electrochemical exfoliation is a novel and effective approach for graphene synthesis, while waste graphite of spent LIBs hold a great potential for nuclear wastewater treatment and electrochemical exfoliation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of environmental radioactivity
环境科学-环境科学
CiteScore
4.70
自引率
13.00%
发文量
209
审稿时长
73 days
期刊介绍:
The Journal of Environmental Radioactivity provides a coherent international forum for publication of original research or review papers on any aspect of the occurrence of radioactivity in natural systems.
Relevant subject areas range from applications of environmental radionuclides as mechanistic or timescale tracers of natural processes to assessments of the radioecological or radiological effects of ambient radioactivity. Papers deal with naturally occurring nuclides or with those created and released by man through nuclear weapons manufacture and testing, energy production, fuel-cycle technology, etc. Reports on radioactivity in the oceans, sediments, rivers, lakes, groundwaters, soils, atmosphere and all divisions of the biosphere are welcomed, but these should not simply be of a monitoring nature unless the data are particularly innovative.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


