级联靶向递送平台增强抗原交叉呈递和STING激活,实现持久的细胞免疫
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
实现强大和持久的细胞免疫仍然是开发亚单位疫苗的一个关键挑战,主要是由于抗原交叉呈递效率低下和免疫激活不足。在这里,我们通过将人血清白蛋白(HSA)与不同链长的脂肪酸偶联来设计一系列纳米乳液。通过系统筛选,鉴定出棕榈酸修饰的纳米乳液是最有效的载体,具有固有的自佐剂特性和较强的细胞免疫应答能力。值得注意的是,这种配方可以实现级联靶向递送,从淋巴结到抗原呈递细胞(APCs),并最终到达内质网(ER)。在模型抗原卵清蛋白(OVA)和干扰素基因刺激剂(STING)激动剂的共同递送后,纳米乳剂促进了抗原的有效交叉呈递和STING途径的细胞内精确激活。这种协同机制显著增强CD8+ T细胞反应,促进持久记忆的形成,从而在小鼠模型中产生强大的抗肿瘤功效。总的来说,本研究提出了一种安全、通用的纳米乳液平台,克服了亚单位疫苗递送的关键障碍,为下一代疫苗设计提供了一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
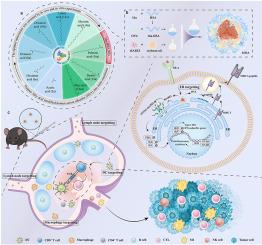
Cascade-targeted delivery platform enhances antigen cross-presentation and STING activation for durable cellular immunity
Achieving robust and durable cellular immunity remains a key challenge in the development of subunit vaccines, primarily due to inefficient antigen cross-presentation and inadequate immune activation. Here, we engineered a series of nano-emulsions by conjugating human serum albumin (HSA) with fatty acids of varying chain lengths. Through systematic screening, the palmitic acid–modified nano-emulsion was identified as the most effective carrier, exhibiting intrinsic self-adjuvant properties and a strong capacity to elicit cellular immune responses. Notably, this formulation enables cascade-targeted delivery, trafficking sequentially from lymph nodes to antigen-presenting cells (APCs), and ultimately to the endoplasmic reticulum (ER). Upon co-delivery of the model antigen ovalbumin (OVA) and a stimulator of interferon genes (STING) agonist, the nano-emulsion facilitates both efficient antigen cross-presentation and precise intracellular activation of the STING pathway. This synergistic mechanism significantly enhances CD8+ T cell responses and promotes durable memory formation, resulting in potent antitumor efficacy in murine models. Collectively, this study presents a safe and versatile nano-emulsion platform that overcomes key barriers in subunit vaccine delivery, offering a promising strategy for next-generation vaccine design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


