通过巨噬细胞极化改善溃疡性结肠炎:靶向hsp90介导的IL-17信号和NF-κB激活
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
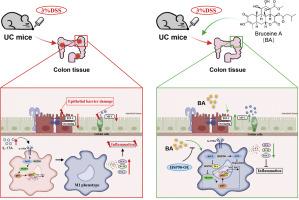
bruceine A (BA)是一种从鸦胆子属植物Brucea javanica果实中提取的天然准藜素类化合物,具有多种药理活性。然而,BA对溃疡性结肠炎(UC)的治疗效果及其潜在的作用机制尚未完全阐明。目的探讨BA对UC的治疗作用并揭示其潜在机制,尤其关注其通过IL-17/NF-κB通路抑制巨噬细胞M1极化的作用。方法用3 %葡聚糖硫酸钠(DSS)建立小鼠UC模型,BA处理。评估结肠长度、组织病理学损伤、炎症介质和上皮屏障完整性,以评估BA的治疗效果。在体外,定量测定细胞活力和巨噬细胞极化生物标志物(如iNOS和TNF-α)来评估BA的细胞保护作用。使用网络药理学研究了BA对抗UC的潜在机制,并通过生物物理方法验证了直接靶点。western blot分析证实BA对UC小鼠和巨噬细胞IL-17/NF-κB通路蛋白表达的调节作用。结果ba通过上调紧密连接蛋白(如ZO-1和Occludin),有效恢复UC小鼠结肠长度,减轻组织病理学炎症,修复上皮屏障。虽然BA不能提高dss损伤的IEC-6细胞的细胞活力,但它能有效抑制THP-1细胞中M1巨噬细胞的极化,这可以通过下调iNOS和TNF-α来证明。机制上,BA与热休克蛋白90 (HSP90)结合,导致IL-17/NF-κB通路相关蛋白的表达显著降低,从而抑制IL-17通路和NF-κB级联激活。值得注意的是,HSP90的过表达消除了BA对IL-17/NF-κB通路和M1巨噬细胞极化的抑制作用。结论sba可有效抑制巨噬细胞M1极化,通过靶向HSP90,进而抑制IL-17和NF-κB通路的激活,从而减轻UC的炎症。这些结果表明,BA是一种很有前途的治疗UC的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bruceine A ameliorates ulcerative colitis via macrophage polarization: Targeting HSP90-mediated IL-17 signaling and NF-κB activation
Background
Bruceine A (BA), a natural quassinoid compound derived from the fruit of Brucea javanica, has demonstrated diverse pharmacological activities. However, the treatment effectiveness of BA against ulcerative colitis (UC) and its underlying mechanisms of action are yet to be fully elucidated.
Purpose
To explore the effects of BA against UC and uncover its underlying mechanisms, with a particular focus on its influence on inhibiting macrophage M1 polarization via the IL-17/NF-κB pathway.
Methods
A murine UC model was constructed using 3 % dextran sulfate sodium (DSS) and treated with BA. Colon length, histopathological damage, inflammatory mediators, and epithelial barrier integrity were assessed to evaluate BA's therapeutic efficacy. In vitro, cell viability and macrophage polarization biomarkers (e.g., iNOS and TNF-α) were quantified to evaluate the cytoprotective effects of BA. The potential mechanisms of BA in combating UC were investigated using network pharmacology, with direct targets validated through biophysical approaches. Furthermore, western blot analysis was employed to confirm BA's regulatory effect on the protein expression of the IL-17/NF-κB pathway in both UC mice and macrophages.
Results
BA treatment effectively restored colon length, attenuated histopathological inflammation, and repaired epithelial barrier via upregulating tight junction proteins (e.g., ZO-1 and Occludin) in UC mice. Although BA failed to enhance cell viability in DSS-injured IEC-6 cells, it potently inhibited M1 macrophage polarization in THP-1 cells, as evidenced by downregulated iNOS and TNF-α. Mechanistically, BA bound to heat shock protein 90 (HSP90), leading to a significant reduction in the expression of IL-17/NF-κB pathway-related proteins, thereby suppressing the IL-17 pathway and the NF-κB cascade activation. Notably, overexpression of HSP90 abrogated BA's inhibition of the IL-17/NF-κB pathway and M1 macrophage polarization.
Conclusions
BA effectively inhibits macrophage M1 polarization, thereby attenuating inflammation in UC by targeting HSP90 and subsequently suppressing the activation of IL-17 and NF-κB pathway. These results imply that BA is a promising therapeutic candidate for UC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


