肝-骨类器官揭示衰老驱动的器官间串扰
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
肝骨轴在与年龄有关的疾病中起着关键作用。然而,目前的模型没有充分捕捉到其复杂的器官间交流。在这里,我们使用工程仿生水凝胶联合阿霉素(DOX)诱导的衰老建立了新的、生理相关的衰老肝脏和骨类器官模型。这些模型成功地再现了标志性的衰老特征:类骨器官矿化减少,衰老标志物升高,类肝器官DNA损伤,结构恶化。值得注意的是,衰老小鼠血清有效地诱导了两种类器官的衰老,证实了系统性衰老调节因子的存在。该平台显示出强大的双向串扰,衰老的类肝器官调节培养基有力地驱动类骨器官的降解,而衰老的类骨器官调节培养基则加剧了类肝器官的功能障碍。在机制上,我们发现27-羟基胆固醇(27-OHC)是一种新的肝细胞源性因子,介导肝-骨通讯。27-OHC不仅会诱导骨类器官衰老,还会与DOX治疗协同加剧骨质流失,这一发现得到了小鼠体内研究的证实,验证了我们的平台在病理损伤背景下的相关性。本研究率先建立了第一个基于类器官的平台,阐明了多器官衰老机制,揭示了27-OHC作为肝-骨轴功能障碍的关键调节因子,并提出了与年龄相关的全身性疾病的新治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
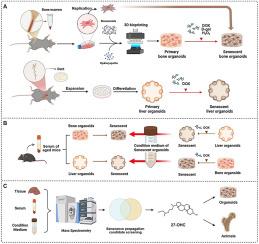
Liver-bone organoids reveal senescence-driven interorgan crosstalk
The liver-bone axis plays a critical role in age-related diseases. However, current models inadequately capture its complex inter-organ communication. Here, we established novel, physiologically relevant senescent liver and bone organoid models using engineered bionic hydrogels combined with doxorubicin (DOX)-induced senescence. These models successfully recapitulated hallmark aging characteristics: bone organoids exhibited reduced mineralization accompanied by elevated senescence markers, and liver organoids demonstrated DNA damage along with structural deterioration. Notably, aged mouse serum effectively induced senescence in both organoid types, confirming the existence of systemic aging regulators. The platform demonstrated robust bidirectional crosstalk, with senescent liver organoid-conditioned medium potently driving degradation in bone organoids and senescent bone organoid-conditioned medium aggravating dysfunction in liver organoids. Mechanistically, we identified 27-hydroxycholesterol (27-OHC) as a novel hepatocyte-derived factor mediating liver-to-bone communication. 27-OHC not only induced bone organoids senescence but also synergized with DOX treatment to exacerbate bone loss, a finding corroborated by in vivo mouse studies that validated the relevance of our platform in the context of pathological damage. This study pioneers the first organoid-based platform that elucidates multi-organ aging mechanisms, uncovering 27-OHC as a pivotal regulator of liver-bone axis dysfunction and proposing novel treatment strategies for age-related systemic disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


