微流控芯片集成血管化子宫内膜复合物:线粒体功能和旁分泌串扰增强再生潜能
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
子宫内膜损伤是一种常见的妇科疾病,对生育和妇女健康构成重大威胁。虽然目前报道的子宫内膜类器官显示出重塑子宫内膜功能的潜力,但它们往往缺乏体内组织的复杂性和生理相关性。在这里,我们介绍了一个血管化的三细胞子宫内膜复合体,将子宫内膜上皮类器官、基质细胞和内皮细胞整合在一个微流控芯片中,该微流控芯片含有含有基质蛋白和纤维蛋白的复合水凝胶。这种新型子宫内膜复合物在子宫内膜损伤的免疫缺陷小鼠模型中表现出强劲的生长和子宫内膜修复能力,显著提高了妊娠率。单细胞RNA测序揭示了血管化子宫内膜复合体中上皮细胞、间质细胞和内皮细胞之间的双向旁分泌串扰。内皮细胞分泌BMP6和Galectin-9,增强线粒体功能,促进上皮细胞增殖。相反,上皮细胞和基质细胞分别分泌WNT7A和WNT5A,刺激内皮细胞血管生成和血管网络形成。这些发现揭示了支撑血管化三细胞子宫内膜复合物优越再生特性的旁分泌相互作用,为子宫内膜修复提供了潜在的治疗策略,并为子宫内膜病理生理研究提供了有价值的体外模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
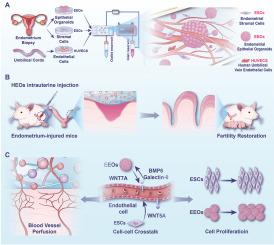
Microfluidic chip-integrated vascularized endometrial complexes: Mitochondrial function and paracrine crosstalk enhance regenerative potential
Endometrial injury is a prevalent gynecological condition that poses a significant threat to fertility and women's health. While the current reported endometrial organoids demonstrate potential in remodeling endometrial functions, they often lack the complexity and physiological relevance of in vivo tissue. Here, we introduce a vascularized triple-cellular endometrial complex integrating endometrial epithelial organoids, stromal cells, and endothelial cells within a microfluidic chip with a composite hydrogel comprising Matrigel and fibrin. This novel endometrial complex exhibits robust growth and endometrial repair capabilities in an immunodeficient mouse model of endometrial damage, significantly improving pregnancy rates. Single-cell RNA sequencing revealed bidirectional cellular paracrine crosstalk between epithelial, stromal, and endothelial cells in the vascularized endometrial complex. Endothelial cells secrete BMP6 and Galectin-9, which enhance mitochondrial function and promote epithelial cell proliferation. Conversely, epithelial and stromal cells secrete WNT7A and WNT5A, respectively, to stimulate angiogenesis and vascular network formation of endothelial cells. These findings reveal the paracrine interactions that underpin the superior regenerative properties of the vascularized triple-cellular endometrial complex, offering a potential therapeutic strategy for endometrial repair and a valuable in vitro model for endometrial pathophysiological studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


