残花生壳作为锌离子生物吸附剂:变量分析、去除机理及处理
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
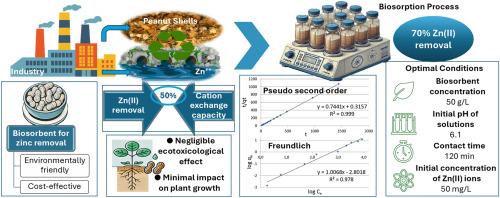
地表水质量的退化是人类活动,特别是工业和农业过程的结果。生物吸附剂被广泛认为是具有成本效益的吸附剂,许多研究已经证明了它们在去除水溶液中的重金属方面的功效。本研究旨在阐明花生壳作为吸附剂去除Zn(II)离子的最佳条件和主要机理。吸附实验采用批处理方式,在不同参数下进行。对生物量进行了一系列的分析技术,以确定其物理化学和环境特性。研究了不同的动力学模型,并用吸附等温线分析了生物吸附过程。最佳脱锌条件为:生物质浓度为50 g/L,初始pH为6.1,接触时间为120 min。使用这些参数,大约84%的金属被去除。此外,受锌污染的花生壳在陆地上处理后,其生态毒理学特性没有显着变化。结果表明,花生壳是一种具有较好应用前景和经济效益的锌离子生物吸附剂,其中阳离子交换机制非常重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Residual peanut shells as zinc ions biosorbent: analysis of variables, removal mechanisms and disposal
The degradation of surface water quality is a consequence of human activities, particularly industrial and agricultural processes. Biosorbents are widely recognised as cost-effective adsorbents and numerous studies have demonstrated their efficacy in removing heavy metals from aqueous solutions. This study aims to elucidate the optimal conditions and the primary mechanisms involved in the removal of Zn(II) ions using ground peanut shells as adsorbent. The adsorption experiments were carried out in batch mode, with different parameter variations. The biomass was subjected to a series of analytical techniques to determine its physicochemical and environmental characteristics. Different kinetic models of the process were studied and adsorption isotherms were used to analyse the biosorption process. The optimal conditions for zinc removal were obtained at a biomass concentration of 50 g/L, an initial pH of 6.1, and a contact time of 120 min. Using these parameters, approximately 84 % of the metal was removed. Furthermore, peanut shells contaminated with zinc do not show significant changes in their ecotoxicological properties when disposed of on land. The results show that peanut shells are a promising and cost-effective alternative biosorbent for the removal of zinc ions from aqueous media, where cation exchange mechanism is of great importance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


