单萜烯在小鼠淋巴瘤试验中的物质消耗:定量、影响和缓解
IF 3.5
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
对于具有不利物理化学性质的测试物质,在体外毒性测试过程中,不同的物质消耗途径,如挥发或吸附到聚合物或血清成分,可以降低生物利用度。如果不加以考虑,这可能会导致毒性被低估,甚至导致假阴性结果。因此,为了获得可靠的结果,需要彻底了解体外测试系统以及潜在的陷阱和物质浓度的分析确认。本文研究了单萜烯(R)-(+)-柠檬烯(RLIM)和β-月桂烯(βMYR)、单萜醇(±)-芳樟醇(LIN)和已知挥发性诱变剂1-溴opopane (1-BP)在小鼠淋巴瘤试验(MLA)中的遗传毒性,并对暴露浓度进行了量化。此外,提出了一个无顶空(HS)的培养装置,允许悬浮细胞与挥发性测试物质充分暴露。RLim、βMYR和1-BP在孵育过程中表现出快速和定量的蒸发,这可能会混淆遗传毒性试验的结果,而在无hs的孵育环境中,这一结果可以最小化。此外,在无hs培养期间,RLIM和βMYR与血清成分的广泛结合显示降低了生物利用度。虽然无hs的培养设置增加了MLA对挥发性基因毒素的敏感性,但未观察到单萜烯的致突变性迹象,强调了它们的安全性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
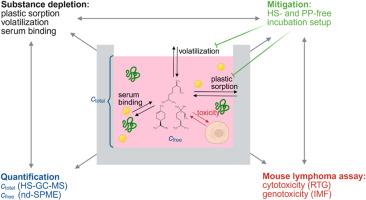
Substance depletion of monoterpenes in the mouse lymphoma assay: Quantification, impact and mitigation
For test substances with unfavorable physicochemical properties, different pathways of substance depletion such as volatilization or sorption to polymers or serum constituents can decrease the bioavailable fraction during in vitro toxicity testing. If not accounted for, this can lead to underestimated toxicity or even false-negative results. Therefore a thorough understanding of the in vitro test system as well as potential pitfalls and analytical confirmation of substance concentrations are required for reliable results. Here, we investigated the genotoxicity of the monoterpenes (R)-(+)-limonene (RLIM) and β-myrcene (βMYR), the monoterpene alcohol (±)-linalool (LIN) and the known volatile mutagen 1-bromopopane (1-BP) in the mouse lymphoma assay (MLA) and quantified the exposure concentrations. Additionally, a headspace (HS)-free incubation setup is presented which allows for sufficient exposure of suspension cells with volatile test substances. RLim, βMYR and 1-BP, showed rapid and quantitative evaporation during incubation, potentially confounding the outcome of the genotoxicity test which could be minimized in the HS-free incubation setup. Furthermore, extensive binding of RLIM and βMYR to serum constituents was shown to decrease bioavailability during HS-free incubation. While the HS-free incubation setup increased the sensitivity of the MLA for volatile genotoxins, no signs for mutagenicity were observed for the monoterpenes, underscoring their safety.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


