长期使用益生菌治疗可减轻衰老加速小鼠的肠道功能障碍和肠道阿尔茨海默病相关病理
IF 4
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
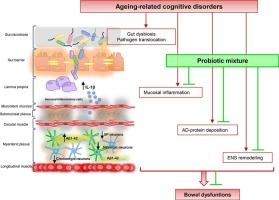
肠道微生物群的操纵代表了一种潜在的治疗策略,以抵消肠道紊乱的患者与年龄相关的认知障碍。在这里,我们研究了一种新型益生菌混合物(PM)在加速衰老小鼠模型中对抗肠道症状和肠道ad病理的作用。SAMP8小鼠口服PM (1 × 109 CFU\AFU株/小鼠/天)或安慰剂治疗2个月。研究了PM对大鼠体内结肠运输和体外结肠收缩活性的影响。测定大鼠胆碱能、快动能和氮能神经元密度以及结肠淀粉样蛋白-β1-42 (Aβ)、HuC/D和IL-1β水平的变化。血浆GABA浓度也进行了研究。SAMP8小鼠显示:1)结肠运输和体外收缩能力受损;2)肌胆碱能神经元、速激能神经元和氮能神经元减少;3)结肠Aβ1-42和IL-1β水平升高,4)循环GABA浓度降低。PM通过使肠道胆碱能、快动能和氮能神经递质正常化,减少肠道ad相关蛋白积累和炎症,恢复体内和体外结肠运动。最后,PM诱导SAMP8小鼠血浆GABA浓度的调节。总的来说,补充PM可能是一种合适的治疗策略,可以对抗与年龄相关的认知障碍相关的肠道紊乱。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Prolonged treatment with probiotics mitigates intestinal dysfunction and enteric Alzheimer's-related pathology in senescence-accelerated mice
The manipulation of gut microbiota represents a potential therapeutic strategy to counteract gut disturbances in patients with age-related cognitive disorders. Here, we investigated the effects of a novel probiotic mixture (PM) in counteracting intestinal symptoms and enteric AD-pathology in an accelerated-senescence mouse model. SAMP8 mice were treated orally with PM at 1 × 109 CFU\AFU strain/mouse/day or placebo for two months. The effects of PM on in vivo colonic transit and in vitro colonic contractile activity were evaluated. Changes in cholinergic, tachykininergic and nitrergic neuron density as well as colonic amyloid-β1–42 (Aβ), HuC/D and IL-1β levels were assessed. Plasma GABA concentrations were also investigated. SAMP8 mice displayed: 1) impairments in colonic transit and in vitro contractility; 2) decrease in myenteric cholinergic, tachykininergic and nitrergic neurons; 3) increase in colonic Aβ1–42 and IL-1β levels and 4) decrease in circulating GABA concentrations. PM restored in vivo and in vitro colonic motility by normalizing enteric cholinergic, tachykininergic and nitrergic neurotransmissions and decreased enteric AD-related protein accumulation and inflammation. Finally, PM induced a modulation of plasma GABA concentrations in SAMP8 mice. Overall, PM supplementation may represent a suitable therapeutic strategy to counteract gut disturbances related to age-related cognitive disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


