大戟地上部的抗炎二萜
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
对大戟(Euphorbia wallichii)地上部分进行了深入的植物化学研究,发现了10种lathyrane型二萜,其中包括6种以前未报道的化合物(1 - 4,6和8)。通过综合光谱分析确定了它们的结构,并根据电子圆二色技术确定了它们的绝对立体化学性质。其中化合物2对NO的抑制活性最好,并通过网络药理学分析探讨其潜在的抗炎机制。进一步实验验证,二萜2可能通过抑制促炎因子、调节PI3K/Akt/mTOR信号通路发挥抗炎作用。简而言之,目前的工作证实了瓦利奇菌作为一种天然抗炎剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
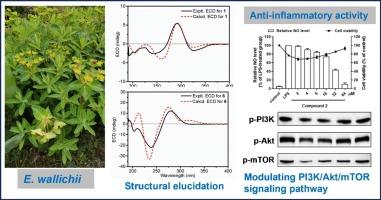
Anti-inflammatory lathyrane diterpenoids from the aerial parts of Euphorbia wallichii
An intensive phytochemical study on the aerial parts of Euphorbia wallichii afforded ten lathyrane-type diterpenoids, including six formerly unreported ones (1–4, 6 and 8). Their structures were determined via comprehensive spectroscopic analyses, and the absolute stereochemistries were assigned on the basis of electronic circular dichroism technique. Among these diterpenoids, compound 2 exhibited the best inhibitory activity on NO production and its potential anti-inflammatory mechanism was then explored by network pharmacology analysis. Further experimental validation demonstrated that diterpenoid 2 could exert its anti-inflammatory effect by suppressing pro-inflammatory factors and modulating PI3K/Akt/mTOR signaling pathway. In short, the present work confirms the promising potential of E. wallichii as a source of natural anti-inflammatory agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


