近红外激光激活PLGA-PDA核壳纳米杂化物在正畸白斑预防中的协同光热抗菌和持续离子释放
IF 5.5
2区 医学
Q1 DENTISTRY, ORAL SURGERY & MEDICINE
引用次数: 0
摘要
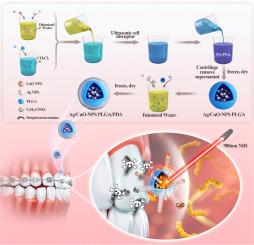
目的制备近红外(NIR)激光激活的包裹Ag/CuO纳米粒子的PLGA-PDA核壳复合材料,同时光热抗菌和持续释放Ag+/Cu2+,并评价其对变形链球菌的杀菌效果和预防正畸白斑病变的潜力。方法采用双乳液和自聚合的方法将sag /CuO纳米颗粒包裹在PLGA/PDA纳米球中。得到的核壳颗粒在组成、尺寸分布、zeta电位、形态、光热性能、释放动力学和细胞相容性等方面进行了全面表征。最后,我们评估了它们在近红外激光照射下对变形链球菌的杀菌效果,并评估了它们预防正畸白斑病变的潜力。结果合成的Ag/CuONPs@PLGA/PDA纳米粒子具有均匀的纳米级分布和稳定的zeta电位。在0.5 W 980 nm近红外照射下,光响应性聚多巴胺涂层迅速将光能转化为热能,使抗菌药物持续释放16天。对变形链球菌具有强效杀菌活性,有效预防正畸白斑病变。至关重要的是,体内分析表明,几乎不存在IL-6表达,VEGF/TGF-β极化上调,增强抗炎反应,促进血管生成和组织再生。主要器官组织病理学检查未见明显炎症,证实了全身生物相容性。结论Ag/CuONPs@PLGA/PDA缓释微球在0.5 W短时间近红外照射下可实现药物控释,有效抑制变形链球菌浮游细胞和牙菌斑生物膜的形成,预防正畸白斑病变。此外,这些微球显著减轻伤口部位的炎症,加速组织再生,并表现出良好的生物相容性。本文报道的Ag/CuONPs@PLGA/PDA核壳纳米杂化物可以很容易地配制成正畸粘接剂或透明矫正器表面的抗菌涂层。在偶尔的近红外激活下,它们提供局部光热“爆发”,同时持续释放Ag + /Cu +,实现有效的、按需根除变形链球菌,同时保护周围组织。通过将这种双模式防御整合到常规器械中,临床医生可以在不影响结合强度或生物相容性的情况下大大减少白斑病变的发生率,最终改善治疗结果和患者满意度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Near-infrared laser-activated PLGA-PDA core-shell nanohybrids for synergistic photothermal antibacterial therapy and sustained ion release in orthodontic white spot lesions prevention
Objective
To develop near-infrared (NIR) laser-activated PLGA–PDA core–shell nanohybrids encapsulating Ag/CuO nanoparticles for simultaneous photothermal antibacterial therapy and sustained Ag+/Cu2+ release, and to evaluate their bactericidal efficacy against Streptococcus mutans and preventive potential in orthodontic white spot lesions.
Methods
Ag/CuO nanoparticles were encapsulated in PLGA/PDA nanospheres via a double‐emulsion and self‐polymerization approach. The resulting core–shell particles were fully characterized for composition, size distribution, zeta potential, morphology, photothermal performance, release kinetics, and cytocompatibility. Finally, we assessed their bactericidal efficacy under NIR laser irradiation against Streptococcus mutans and evaluated their potential to prevent orthodontic white‐spot lesions.
Results
Synthesized Ag/CuO![]() NPs@PLGA/PDA nanoparticles exhibit uniform nanoscale distribution and stable zeta potential. Upon 0.5 W 980-nm NIR irradiation, the photoresponsive polydopamine coating rapidly converts light to thermal energy, enabling sustained antibacterial drug release for 16 days. This confers potent bactericidal activity against Streptococcus mutans, effectively preventing orthodontic white spot lesions. Crucially, in vivo analysis demonstrates near-absent IL-6 expression with polarized VEGF/TGF-β upregulation, enhancing anti-inflammatory responses while promoting angiogenesis and tissue regeneration. Histopathological examination of major organs reveals no significant inflammation, confirming systemic biocompatibility.
NPs@PLGA/PDA nanoparticles exhibit uniform nanoscale distribution and stable zeta potential. Upon 0.5 W 980-nm NIR irradiation, the photoresponsive polydopamine coating rapidly converts light to thermal energy, enabling sustained antibacterial drug release for 16 days. This confers potent bactericidal activity against Streptococcus mutans, effectively preventing orthodontic white spot lesions. Crucially, in vivo analysis demonstrates near-absent IL-6 expression with polarized VEGF/TGF-β upregulation, enhancing anti-inflammatory responses while promoting angiogenesis and tissue regeneration. Histopathological examination of major organs reveals no significant inflammation, confirming systemic biocompatibility.
Conclusion
Triggered by brief near-infrared irradiation (0.5 W), Ag/CuO![]() NPs@PLGA/PDA sustained-release microspheres enable controlled drug release that effectively suppresses both Streptococcus mutans planktonic cells and dental plaque biofilm formation, thereby preventing orthodontic white spot lesions. Furthermore, these microspheres significantly attenuate wound-site inflammation, accelerate tissue regeneration, and demonstrate excellent biocompatibility.
NPs@PLGA/PDA sustained-release microspheres enable controlled drug release that effectively suppresses both Streptococcus mutans planktonic cells and dental plaque biofilm formation, thereby preventing orthodontic white spot lesions. Furthermore, these microspheres significantly attenuate wound-site inflammation, accelerate tissue regeneration, and demonstrate excellent biocompatibility.
Clinical Significance
The Ag/CuO![]() NPs@PLGA/PDA core–shell nanohybrids reported here can be readily formulated into antimicrobial coatings for orthodontic adhesives or clear aligner surfaces. Upon occasional NIR activation, they deliver a localized photothermal “burst” together with sustained Ag⁺/Cu²⁺ release, achieving potent, on-demand eradication of Streptococcus mutans while preserving surrounding tissues. By integrating this dual-mode defense into routine appliances, clinicians may substantially reduce the incidence of white spot lesions without compromising bonding strength or biocompatibility, ultimately improving treatment outcomes and patient satisfaction.
NPs@PLGA/PDA core–shell nanohybrids reported here can be readily formulated into antimicrobial coatings for orthodontic adhesives or clear aligner surfaces. Upon occasional NIR activation, they deliver a localized photothermal “burst” together with sustained Ag⁺/Cu²⁺ release, achieving potent, on-demand eradication of Streptococcus mutans while preserving surrounding tissues. By integrating this dual-mode defense into routine appliances, clinicians may substantially reduce the incidence of white spot lesions without compromising bonding strength or biocompatibility, ultimately improving treatment outcomes and patient satisfaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of dentistry
医学-牙科与口腔外科
CiteScore
7.30
自引率
11.40%
发文量
349
审稿时长
35 days
期刊介绍:
The Journal of Dentistry has an open access mirror journal The Journal of Dentistry: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
The Journal of Dentistry is the leading international dental journal within the field of Restorative Dentistry. Placing an emphasis on publishing novel and high-quality research papers, the Journal aims to influence the practice of dentistry at clinician, research, industry and policy-maker level on an international basis.
Topics covered include the management of dental disease, periodontology, endodontology, operative dentistry, fixed and removable prosthodontics, dental biomaterials science, long-term clinical trials including epidemiology and oral health, technology transfer of new scientific instrumentation or procedures, as well as clinically relevant oral biology and translational research.
The Journal of Dentistry will publish original scientific research papers including short communications. It is also interested in publishing review articles and leaders in themed areas which will be linked to new scientific research. Conference proceedings are also welcome and expressions of interest should be communicated to the Editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


