超高速高稳定钠离子电池用低价多元素协同稳定双金属普鲁士蓝阴极
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
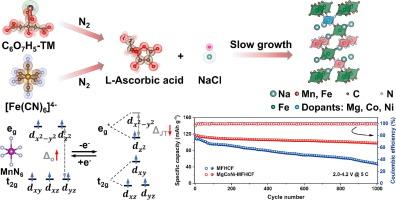
具有双金属反应中心的普鲁士蓝类似物(PBA)阴极的合理设计是高能钠离子电池(sib)的基础策略,但其电化学性能受到结构不稳定和动力学缓慢的固有限制。在此,我们提出了一种多元素共掺杂策略,通过用MgII、CoII和NiII取代n配位位点来实现双金属Na2Mn0.5Fe0.5[Fe(CN)6] (MFHCF)的整体优化。具体来说,MgCoNi-MFHCF提供了卓越的倍率能力(分别在0.1和30℃下为145.9和85.3 mAh g - 1),出色的循环稳定性(1000次循环后容量保持率为83.1%)和高能量密度(满电池时为304.5 Wh kg - 1)。原位/非原位技术和理论计算表明,MgCoNi-MFHCF经历了可逆的三相转变,其体积收缩/膨胀减轻,这源于Jahn-Teller畸变的减轻。认为阳离子掺杂通过稳定过渡金属配位环境,提高了氧化还原反应的可逆性,同时减小了带隙,降低了离子扩散能垒,从而加速了电化学动力学。本研究建立了具有双金属反应中心的高性能sib正极材料的通用多元素工程策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Low-valent multielement synergy stabilizing bimetallic Prussian blue cathode for ultra-fast and highly stable sodium-ion batteries
The rational design of Prussian blue analogue (PBA) cathodes with bimetallic reaction centers represents a cornerstone strategy for high-energy sodium-ion batteries (SIBs), yet their electrochemical performance is inherently limited by structural instability and sluggish kinetics. Herein, we propose a multielement co-doping strategy to achieve a holistic optimization of bimetallic Na2Mn0.5Fe0.5[Fe(CN)6] (MFHCF) by substituting N-coordinated sites with MgII, CoII, and NiII. Specifically, the MgCoNi-MFHCF delivers a superior rate capability (145.9 and 85.3 mAh g−1 under 0.1 and 30 C, respectively), outstanding cycling stability (83.1% capacity retention over 1000 cycles), and high energy density (304.5 Wh kg−1 for the full cell). In situ/ex situ techniques and theoretical calculations reveal that the MgCoNi-MFHCF experiences a reversible tri-phase transition with mitigated volume contraction/expansion, which originates from the alleviation of the Jahn-Teller distortion. It is considered that the cation doping enhances redox reaction reversibility through stabilized transition-metal coordination environments while reducing bandgaps and lowering ionic diffusion energy barrier, leading to accelerated electrochemical kinetics. This study establishes a generalizable multielement engineering strategy for high-performance cathode materials with bimetallic reaction centers for SIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


