气化后铁焦碳结构对其燃烧行为和动力学影响机理的分子研究:实验,ReaxFF MD,和DFT
IF 7.7
2区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
铁焦作为一种低碳炼铁燃料,因其高反应性和资源高效利用而备受关注。然而,气化后铁焦的结构特征及其对后续燃烧的影响机理尚不清楚。本研究利用XRD和拉曼光谱研究了气化对铁焦碳结构的影响,并结合热重分析、ReaxFF MD和DFT计算揭示了碳结构对燃烧行为和动力学的影响机理。结果表明,铁/铁氧化物催化的气化反应导致铁焦碳结构缺陷增多。气化程度越高的铁焦,其后续燃烧反应活性越大。在非等温燃烧过程中,提高加热速率可以显著提高焦炭的燃烧性能。ReaxFF MD模拟表明,氧自由基优先攻击碳结构中的空位缺陷并与之发生反应,这是缺陷结构反应活性增加的主要原因。由于碳层之间的卷曲效应,燃烧时的活化能随着碳转化率的升高先升高后降低。DFT计算表明,碳结构中的空位缺陷对提高燃烧性能起着至关重要的作用。一方面,增加的缺陷提供了更多的活性位点,降低了O2分子的吸附能。另一方面,缺陷碳结构中的范德华相互作用和化学键的协同作用有效地降低了燃烧反应的活化能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
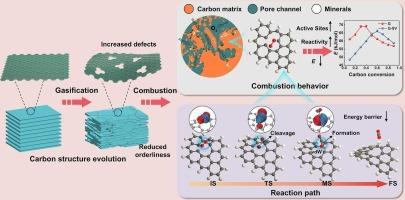
Molecular insights into the influence mechanism of carbon structure in iron coke after gasification on its combustion behavior and kinetics: Experiments, ReaxFF MD, and DFT
Iron coke has attracted attention as a low-carbon ironmaking fuel due to its high reactivity and efficient resource utilization. However, the structural characteristics of iron coke after gasification and their effect mechanisms affecting subsequent combustion remain unclear. This study investigated the effects of gasification on the carbon structure of iron coke using XRD and Raman spectroscopy, and revealed the influence mechanism of carbon structure on combustion behavior and kinetics through combined thermogravimetric analysis, ReaxFF MD, and DFT calculations. The results demonstrate that the gasification reaction catalyzed by iron/iron oxides induces more defects in the carbon structure of iron coke. The higher the gasification degree of iron coke, the greater its following combustion reactivity. Increasing the heating rate in the non-isothermal combustion process can markedly enhance the combustion performance of iron coke. ReaxFF MD simulations reveal that oxygen radicals preferentially attack and react with vacancy defects in the carbon structure, which is the primary reason for the increased reactivity of defective structures. Due to the curling effect between carbon layers, the activation energy during combustion initially increases and then decreases with rising carbon conversion. DFT calculations indicate that vacancy defects in the carbon structure play a critical role in enhancing combustion behavior. On one hand, the increased defects provide more active sites, reducing the adsorption energy for O2 molecules. On the other hand, the synergistic effect of van der Waals interactions and chemical bonds in defective carbon structures effectively reduces activation energy for the combustion reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel Processing Technology
工程技术-工程:化工
CiteScore
13.20
自引率
9.30%
发文量
398
审稿时长
26 days
期刊介绍:
Fuel Processing Technology (FPT) deals with the scientific and technological aspects of converting fossil and renewable resources to clean fuels, value-added chemicals, fuel-related advanced carbon materials and by-products. In addition to the traditional non-nuclear fossil fuels, biomass and wastes, papers on the integration of renewables such as solar and wind energy and energy storage into the fuel processing processes, as well as papers on the production and conversion of non-carbon-containing fuels such as hydrogen and ammonia, are also welcome. While chemical conversion is emphasized, papers on advanced physical conversion processes are also considered for publication in FPT. Papers on the fundamental aspects of fuel structure and properties will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


